New clothes for the jasmonic acid receptor COI1: delayed abscission, meristem arrest and apical dominance
- PMID: 23573263
- PMCID: PMC3613422
- DOI: 10.1371/journal.pone.0060505
New clothes for the jasmonic acid receptor COI1: delayed abscission, meristem arrest and apical dominance
Erratum in
-
Correction: New clothes for the jasmonic acid receptor COI1: delayed abscission, meristem arrest and apical dominance.PLoS One. 2015 Mar 25;10(3):e0119063. doi: 10.1371/journal.pone.0119063. eCollection 2015. PLoS One. 2015. PMID: 25807396 Free PMC article. No abstract available.
Abstract
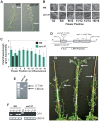
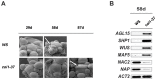
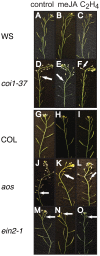
In a screen for delayed floral organ abscission in Arabidopsis, we have identified a novel mutant of CORONATINE INSENSITIVE 1 (COI1), the F-box protein that has been shown to be the jasmonic acid (JA) co-receptor. While JA has been shown to have an important role in senescence, root development, pollen dehiscence and defense responses, there has been little focus on its critical role in floral organ abscission. Abscission, or the detachment of organs from the main body of a plant, is an essential process during plant development and a unique type of cell separation regulated by endogenous and exogenous signals. Previous studies have indicated that auxin and ethylene are major plant hormones regulating abscission; and here we show that regulation of floral organ abscission is also controlled by jasmonic acid in Arabidopsis thaliana. Our characterization of coi1-1 and a novel allele (coi1-37) has also revealed an essential role in apical dominance and floral meristem arrest. In this study we provide genetic evidence indicating that delayed abscission 4 (dab4-1) is allelic to coi1-1 and that meristem arrest and apical dominance appear to be evolutionarily divergent functions for COI1 that are governed in an ecotype-dependent manner. Further characterizations of ethylene and JA responses of dab4-1/coi1-37 also provide new information suggesting separate pathways for ethylene and JA that control both floral organ abscission and hypocotyl growth in young seedlings. Our study opens the door revealing new roles for JA and its interaction with other hormones during plant development.
Conflict of interest statement
Figures



Similar articles
-
Four shades of detachment: regulation of floral organ abscission.Plant Signal Behav. 2014;9(11):e976154. doi: 10.4161/15592324.2014.976154. Plant Signal Behav. 2014. PMID: 25482787 Free PMC article. Review.
-
Jasmonic Acid Inhibits Auxin-Induced Lateral Rooting Independently of the CORONATINE INSENSITIVE1 Receptor.Plant Physiol. 2018 Aug;177(4):1704-1716. doi: 10.1104/pp.18.00357. Epub 2018 Jun 22. Plant Physiol. 2018. PMID: 29934297 Free PMC article.
-
The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis.Plant Cell. 2011 Mar;23(3):1000-13. doi: 10.1105/tpc.111.083089. Epub 2011 Mar 29. Plant Cell. 2011. PMID: 21447791 Free PMC article.
-
A conditionally fertile coi1 allele indicates cross-talk between plant hormone signalling pathways in Arabidopsis thaliana seeds and young seedlings.Planta. 2002 Aug;215(4):549-56. doi: 10.1007/s00425-002-0787-4. Epub 2002 Jul 4. Planta. 2002. PMID: 12172836
-
Plant oxylipins: COI1/JAZs/MYC2 as the core jasmonic acid-signalling module.FEBS J. 2009 Sep;276(17):4682-92. doi: 10.1111/j.1742-4658.2009.07194.x. Epub 2009 Aug 3. FEBS J. 2009. PMID: 19663905 Review.
Cited by
-
Jasmonate Signaling Pathway Modulates Plant Defense, Growth, and Their Trade-Offs.Int J Mol Sci. 2022 Apr 1;23(7):3945. doi: 10.3390/ijms23073945. Int J Mol Sci. 2022. PMID: 35409303 Free PMC article. Review.
-
Hydrogen sulfide inhibits ethylene-induced petiole abscission in tomato (Solanum lycopersicum L.).Hortic Res. 2020 Feb 1;7:14. doi: 10.1038/s41438-019-0237-0. eCollection 2020. Hortic Res. 2020. PMID: 32025317 Free PMC article.
-
To grow old: regulatory role of ethylene and jasmonic acid in senescence.Front Plant Sci. 2015 Jan 29;6:20. doi: 10.3389/fpls.2015.00020. eCollection 2015. Front Plant Sci. 2015. PMID: 25688252 Free PMC article.
-
Transcriptional Regulation of Abscission Zones.Plants (Basel). 2019 Jun 6;8(6):154. doi: 10.3390/plants8060154. Plants (Basel). 2019. PMID: 31174352 Free PMC article. Review.
-
Correction: New clothes for the jasmonic acid receptor COI1: delayed abscission, meristem arrest and apical dominance.PLoS One. 2015 Mar 25;10(3):e0119063. doi: 10.1371/journal.pone.0119063. eCollection 2015. PLoS One. 2015. PMID: 25807396 Free PMC article. No abstract available.
References
-
- Addicott FT (1982) Abscission. Berkeley, CA:.University of California Press.
-
- Binder B, Patterson S (2009) Ethylene-dependent and -independent regulation of abscission. Stewart Postharvest Reviews
-
- Brown KM (1997) Ethylene and abscission. Physiol Plant 100: 567–576.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

