Tacrolimus inhibits NF-κB activation in peripheral human T cells
- PMID: 23573283
- PMCID: PMC3613409
- DOI: 10.1371/journal.pone.0060784
Tacrolimus inhibits NF-κB activation in peripheral human T cells
Abstract
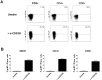
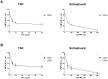
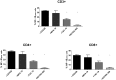
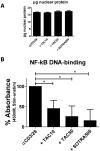
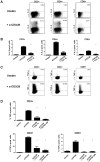
The calcineurin inhibitor, tacrolimus (TAC), inhibits the protein phosphatase activity of calcineurin, leading to suppression of the nuclear translocation of NFAT and inhibition of T cell activation. Apart from NFAT also the transcription factor NF-κB plays a key functional role in T cell activation. Therefore, blockade of the NF-κB activation cascade by immunosuppressive drugs prevents immune activation. Here we studied whether TAC blocks NF-κB activation in peripheral human T cells. After anti-CD3/CD28-activation of T cells from healthy volunteers, NF-κB (p65) phosphorylation was measured by flow cytometry in CD3+ T cells, CD4+ helper T cells and CD8+ cytotoxic T cells in the absence and presence of TAC 10 ng/mL, sotrastaurin 500 nM (positive control) and mycophenolic acid 10 µg/mL (negative control; n = 6). NF-κB transcriptional activity was measured by ELISA and intracellular TNFα protein, a downstream target, was measured by flow cytometry to assess the functional consequences of NF-κB blockade. Anti-CD3/28-activation induced NF-κB phosphorylation in CD3+ T cells, CD4+ T cells and CD8+ T cells by 34% (mean), 38% and 30% resp. (p<0.01). Sotrastaurin inhibited NF-κB activation in the respective T cell subsets by 93%, 95% and 86% (p<0.01 vs. no drug), while mycophenolic acid did not affect this activation pathway. Surprisingly, TAC also inhibited NF-κB phosphorylation, by 55% (p<0.01) in CD3+ T cells, by 56% (p<0.01) in CD4+ T cells and by 51% in CD8+ T cells (p<0.01). In addition, TAC suppressed NF-κB DNA binding capacity by 55% (p<0.05) in CD3+ T cells and TNFα protein expression was inhibited in CD3+ T cells, CD4+ T cells and CD8+ T cells by 76%, 71% and 93% resp. (p<0.01 vs. no drug), confirming impaired NF-κB signaling. This study shows the suppressive effect of TAC on NF-κB signaling in peripheral human T cell subsets, measured at three specific positions in the NF-κB activation cascade.
Conflict of interest statement
Figures
References
-
- Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, et al. (2006) Immunosuppression: evolution in practice and trends, 1994-2004. Am J Transplant 6: 1111–1131. - PubMed
-
- Cecka JM (2005) The OPTN/UNOS Renal Transplant Registry. Clin Transpl: 1-16. - PubMed
-
- Tang IY, Meier-Kriesche HU, Kaplan B (2007) Immunosuppressive strategies to improve outcomes of kidney transplantation. Semin Nephrol 27: 377–392. - PubMed
-
- Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, et al. (2007) Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575. - PubMed
-
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, et al. (2007) Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR Study. Am J Transplant 7: 560–570. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

