Overexpression of phyA and appA genes improves soil organic phosphorus utilisation and seed phytase activity in Brassica napus
- PMID: 23573285
- PMCID: PMC3616117
- DOI: 10.1371/journal.pone.0060801
Overexpression of phyA and appA genes improves soil organic phosphorus utilisation and seed phytase activity in Brassica napus
Abstract
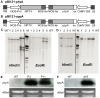
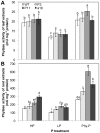
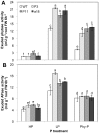
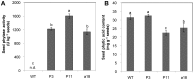
Phytate is the major storage form of organic phosphorus in soils and plant seeds, and phosphorus (P) in this form is unavailable to plants or monogastric animals. In the present study, the phytase genes phyA and appA were introduced into Brassica napus cv Westar with a signal peptide sequence and CaMV 35S promoter, respectively. Three independent transgenic lines, P3 and P11 from phyA and a18 from appA, were selected. The three transgenic lines exhibited significantly higher exuded phytase activity when compared to wild-type (WT) controls. A quartz sand culture experiment demonstrated that transgenic Brassica napus had significantly improved P uptake and plant biomass. A soil culture experiment revealed that seed yields of transgenic lines P11 and a18 increased by 20.9% and 59.9%, respectively, when compared to WT. When phytate was used as the sole P source, P accumulation in seeds increased by 20.6% and 46.9% with respect to WT in P11 and a18, respectively. The P3 line accumulated markedly more P in seeds than WT, while no significant difference was observed in seed yields when phytate was used as the sole P source. Phytase activities in transgenic canola seeds ranged from 1,138 to 1,605 U kg(-1) seeds, while no phytase activity was detected in WT seeds. Moreover, phytic acid content in P11 and a18 seeds was significantly lower than in WT. These results introduce an opportunity for improvement of soil and seed phytate-P bioavailability through genetic manipulation of oilseed rape, thereby increasing plant production and P nutrition for monogastric animals.
Conflict of interest statement
Figures

Similar articles
-
Genetically modified phytase crops role in sustainable plant and animal nutrition and ecological development: a review.3 Biotech. 2017 Jul;7(3):195. doi: 10.1007/s13205-017-0797-3. Epub 2017 Jun 30. 3 Biotech. 2017. PMID: 28667635 Free PMC article. Review.
-
Quantitative conversion of phytate to inorganic phosphorus in soybean seeds expressing a bacterial phytase.Plant Physiol. 2008 Feb;146(2):468-77. doi: 10.1104/pp.107.113480. Epub 2007 Dec 27. Plant Physiol. 2008. PMID: 18162589 Free PMC article.
-
Transgenic maize plants expressing a fungal phytase gene.Transgenic Res. 2008 Aug;17(4):633-43. doi: 10.1007/s11248-007-9138-3. Epub 2007 Oct 12. Transgenic Res. 2008. PMID: 17932782
-
Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate.Plant J. 2001 Mar;25(6):641-9. doi: 10.1046/j.1365-313x.2001.00998.x. Plant J. 2001. PMID: 11319031
-
Rapeseed species and environmental concerns related to loss of seeds of genetically modified oilseed rape in Japan.GM Crops. 2010 May-Jun;1(3):143-56. doi: 10.4161/gmcr.1.3.12761. GM Crops. 2010. PMID: 21844669 Review.
Cited by
-
Harnessing plant-microbe interactions for enhancing farm productivity.Bioengineered. 2014 Jan-Feb;5(1):5-9. doi: 10.4161/bioe.25320. Epub 2013 Jun 12. Bioengineered. 2014. PMID: 23799872 Free PMC article.
-
Characterization of the Catalytic Structure of Plant Phytase, Protein Tyrosine Phosphatase-Like Phytase, and Histidine Acid Phytases and Their Biotechnological Applications.Enzyme Res. 2018 Mar 11;2018:8240698. doi: 10.1155/2018/8240698. eCollection 2018. Enzyme Res. 2018. PMID: 29713527 Free PMC article. Review.
-
Bioprocess for Production, Characteristics, and Biotechnological Applications of Fungal Phytases.Front Microbiol. 2020 Feb 14;11:188. doi: 10.3389/fmicb.2020.00188. eCollection 2020. Front Microbiol. 2020. PMID: 32117182 Free PMC article. Review.
-
Fungal phytases: from genes to applications.Braz J Microbiol. 2020 Sep;51(3):1009-1020. doi: 10.1007/s42770-020-00289-y. Epub 2020 May 14. Braz J Microbiol. 2020. PMID: 32410091 Free PMC article. Review.
-
Genetically modified phytase crops role in sustainable plant and animal nutrition and ecological development: a review.3 Biotech. 2017 Jul;7(3):195. doi: 10.1007/s13205-017-0797-3. Epub 2017 Jun 30. 3 Biotech. 2017. PMID: 28667635 Free PMC article. Review.
References
-
- Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157: 423–447. - PubMed
-
- Dalal RC (1977) Soil organic phosphorus. Adv Agron 29: 83–117.
-
- Brinch-Pedersen H, Sørensen LD, Holm PB (2002) Engineering crop plants: getting a handle on phosphate. Trends Plant Sci 7: 118–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

