Drosophila signal peptidase complex member Spase12 is required for development and cell differentiation
- PMID: 23573290
- PMCID: PMC3616019
- DOI: 10.1371/journal.pone.0060908
Drosophila signal peptidase complex member Spase12 is required for development and cell differentiation
Abstract
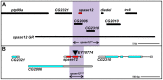
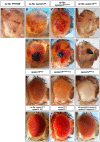
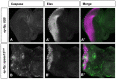
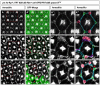
It is estimated that half of all proteins expressed in eukaryotic cells are transferred across or into at least one cellular membrane to reach their functional location. Protein translocation into the endoplasmic reticulum (ER) is critical to the subsequent localization of secretory and transmembrane proteins. A vital component of the translocation machinery is the signal peptidase complex (SPC)--which is conserved from yeast to mammals--and functions to cleave the signal peptide sequence (SP) of secretory and membrane proteins entering the ER. Failure to cleave the SP, due to mutations that abolish the cleavage site or reduce SPC function, leads to the accumulation of uncleaved proteins in the ER that cannot be properly localized resulting in a wide range of defects depending on the protein(s) affected. Despite the obvious importance of the SPC, in vivo studies investigating its function in a multicellular organism have not been reported. The Drosophila SPC comprises four proteins: Spase18/21, Spase22/23, Spase25 and Spase12. Spc1p, the S. cerevisiae homolog of Spase12, is not required for SPC function or viability; Drosophila spase12 null alleles, however, are embryonic lethal. The data presented herein show that spase12 LOF clones disrupt development of all tissues tested including the eye, wing, leg, and antenna. In the eye, spase12 LOF clones result in a disorganized eye, defective cell differentiation, ectopic interommatidial bristles, and variations in support cell size, shape, number, and distribution. In addition, spase12 mosaic tissue is susceptible to melanotic mass formation suggesting that spase12 LOF activates immune response pathways. Together these data demonstrate that spase12 is an essential gene in Drosophila where it functions to mediate cell differentiation and development. This work represents the first reported in vivo analysis of a SPC component in a multicellular organism.
Conflict of interest statement
Figures


Similar articles
-
Three distinct roles for notch in Drosophila R7 photoreceptor specification.PLoS Biol. 2011 Aug;9(8):e1001132. doi: 10.1371/journal.pbio.1001132. Epub 2011 Aug 23. PLoS Biol. 2011. PMID: 21886484 Free PMC article.
-
Inhibition of signal peptidase complex expression affects the development and survival of Schistosoma japonicum.Front Cell Infect Microbiol. 2023 Mar 3;13:1136056. doi: 10.3389/fcimb.2023.1136056. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36936776 Free PMC article.
-
The Drosophila T-box transcription factor Midline functions within the Notch-Delta signaling pathway to specify sensory organ precursor cell fates and regulates cell survival within the eye imaginal disc.Mech Dev. 2013 Nov-Dec;130(11-12):577-601. doi: 10.1016/j.mod.2013.08.001. Epub 2013 Aug 17. Mech Dev. 2013. PMID: 23962751 Free PMC article.
-
Genetic complementation in yeast reveals functional similarities between the catalytic subunits of mammalian signal peptidase complex.J Biol Chem. 2003 Dec 19;278(51):50932-9. doi: 10.1074/jbc.M307542200. Epub 2003 Oct 14. J Biol Chem. 2003. PMID: 14559916
-
NOTCH and the patterning of ommatidial founder cells in the developing Drosophila eye.Results Probl Cell Differ. 2002;37:35-58. doi: 10.1007/978-3-540-45398-7_4. Results Probl Cell Differ. 2002. PMID: 25707068 Review. No abstract available.
Cited by
-
Decoding Alzheimer's Disease With Depression: Molecular Insights and Therapeutic Target.J Cell Mol Med. 2025 Mar;29(5):e70454. doi: 10.1111/jcmm.70454. J Cell Mol Med. 2025. PMID: 40074694 Free PMC article.
-
Differential Proteomics Analysis Unraveled Mechanisms of Arma chinensis Responding to Improved Artificial Diet.Insects. 2022 Jul 2;13(7):605. doi: 10.3390/insects13070605. Insects. 2022. PMID: 35886781 Free PMC article.
-
Ecdysone Receptor Agonism Leading to Lethal Molting Disruption in Arthropods: Review and Adverse Outcome Pathway Development.Environ Sci Technol. 2017 Apr 18;51(8):4142-4157. doi: 10.1021/acs.est.7b00480. Epub 2017 Apr 10. Environ Sci Technol. 2017. PMID: 28355071 Free PMC article. Review.
-
Genetic Variation in Trophic Avoidance Behaviour Shows Fruit Flies are Generally Attracted to Bacterial Substrates.Ecol Evol. 2024 Nov 10;14(11):e70541. doi: 10.1002/ece3.70541. eCollection 2024 Nov. Ecol Evol. 2024. PMID: 39524313 Free PMC article.
-
Spc1 regulates the signal peptidase-mediated processing of membrane proteins.J Cell Sci. 2021 Jul 1;134(13):jcs258936. doi: 10.1242/jcs.258936. Epub 2021 Jul 9. J Cell Sci. 2021. PMID: 34125229 Free PMC article.
References
-
- Paetzel M, Karla A, Strynadka NC, Dalbey RE (2002) Signal peptidases. Chem Rev 102: 4549–4580. - PubMed
-
- von Heijne G (1985) Signal sequences. The limits of variation. J Mol Biol 184: 99–105. - PubMed
-
- Fang H, Panzner S, Mullins C, Hartmann E, Green N (1996) The homologue of mammalian SPC12 is important for efficient signal peptidase activity in Saccharomyces cerevisiae. J Biol Chem 271: 16460–16465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

