An fMRI-based neurologic signature of physical pain
- PMID: 23574118
- PMCID: PMC3691100
- DOI: 10.1056/NEJMoa1204471
An fMRI-based neurologic signature of physical pain
Abstract
Background: Persistent pain is measured by means of self-report, the sole reliance on which hampers diagnosis and treatment. Functional magnetic resonance imaging (fMRI) holds promise for identifying objective measures of pain, but brain measures that are sensitive and specific to physical pain have not yet been identified.
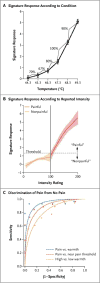
Methods: In four studies involving a total of 114 participants, we developed an fMRI-based measure that predicts pain intensity at the level of the individual person. In study 1, we used machine-learning analyses to identify a pattern of fMRI activity across brain regions--a neurologic signature--that was associated with heat-induced pain. The pattern included the thalamus, the posterior and anterior insulae, the secondary somatosensory cortex, the anterior cingulate cortex, the periaqueductal gray matter, and other regions. In study 2, we tested the sensitivity and specificity of the signature to pain versus warmth in a new sample. In study 3, we assessed specificity relative to social pain, which activates many of the same brain regions as physical pain. In study 4, we assessed the responsiveness of the measure to the analgesic agent remifentanil.
Results: In study 1, the neurologic signature showed sensitivity and specificity of 94% or more (95% confidence interval [CI], 89 to 98) in discriminating painful heat from nonpainful warmth, pain anticipation, and pain recall. In study 2, the signature discriminated between painful heat and nonpainful warmth with 93% sensitivity and specificity (95% CI, 84 to 100). In study 3, it discriminated between physical pain and social pain with 85% sensitivity (95% CI, 76 to 94) and 73% specificity (95% CI, 61 to 84) and with 95% sensitivity and specificity in a forced-choice test of which of two conditions was more painful. In study 4, the strength of the signature response was substantially reduced when remifentanil was administered.
Conclusions: It is possible to use fMRI to assess pain elicited by noxious heat in healthy persons. Future studies are needed to assess whether the signature predicts clinical pain. (Funded by the National Institute on Drug Abuse and others.).
Figures


Comment in
-
Pain, heat, and emotion with functional MRI.N Engl J Med. 2013 Apr 11;368(15):1447-9. doi: 10.1056/NEJMe1213074. N Engl J Med. 2013. PMID: 23574124 No abstract available.
-
A brain signature for acute pain.Trends Cogn Sci. 2013 Jul;17(7):309-10. doi: 10.1016/j.tics.2013.05.001. Epub 2013 Jun 5. Trends Cogn Sci. 2013. PMID: 23747083 Free PMC article.
-
Can pain be measured objectively?Neurosurgery. 2013 Aug;73(2):N24-5. doi: 10.1227/01.neu.0000432627.18847.8e. Neurosurgery. 2013. PMID: 23867275 No abstract available.
References
-
- Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011. - PubMed
-
- Flor H, Turk D. Chronic pain: an integrated biobehavioral approach. Seattle: IASP Press; 2011.
-
- Wager TD, Fields H. Placebo analgesia. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s textbook of pain. Oxford, United Kingdom: Churchill Livingstone; (in press)
-
- Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning: data mining, inference, and prediction. 2. New York: Springer; 2009.
-
- Mitchell T. Does machine learning really work? AI Magazine. 1997;18:11.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
