Altered migratory behavior of interneurons in a model of cortical dysplasia: the influence of elevated GABAA activity
- PMID: 23574639
- PMCID: PMC4128700
- DOI: 10.1093/cercor/bht073
Altered migratory behavior of interneurons in a model of cortical dysplasia: the influence of elevated GABAA activity
Abstract
Appropriate function of the neocortex depends on timely generation and migration of cells produced in the germinal zones of the neocortex and ganglionic eminence (GE). Failure to accurately complete migration results in cortical dysplasia, a developmental syndrome implicated in many neurologic disorders. We developed a model of cortical dysplasia in ferrets involving administration of methylaxozymethanol acetate (MAM), an antimitotic, to pregnant ferrets on gestational day 33, leading to dramatic reduction of layer 4 in the neocortex. Here, using time-lapse video imaging, we investigate dynamic behavior of migrating cells arising from the GE and cortical ventricular zone (CVZ) in ferrets and the role of GABAA activity. Treatment with MAM significantly reduced migration speed and the relative proportion of cells arising from the GE demonstrating exploratory behavior. To a lesser extent, the behavior of cells leaving the CVZ was affected. Pharmacologic inhibition of GABAA receptors (GABAAR) improved the speed of migration and exploratory ability of migrating MAM-treated cells arising from the GE. Additionally, the expression of α2 and α3 subunits of GABAAR and the potassium chloride co-transporter (KCC2) increased in the neocortex of MAM-treated animals. After MAM treatment, increases in endogenous KCC2 and GABAAR combine to alter the dynamic properties and exploratory behavior of migrating interneurons in ferrets. We show a direct correlation between increased GABAA and KCC2 expression with impaired migration and ability to explore the environment.
Keywords: KCC2; MAM; development; ferret; neuronal migration.
Published by Oxford University Press 2013. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures
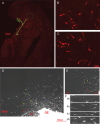



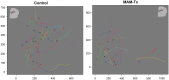
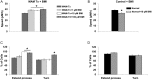


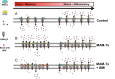
Similar articles
-
Targeted disruption of layer 4 during development increases GABAA receptor neurotransmission in the neocortex.J Neurophysiol. 2014 Jan;111(2):323-35. doi: 10.1152/jn.00652.2012. Epub 2013 Oct 23. J Neurophysiol. 2014. PMID: 24155012 Free PMC article.
-
Alteration of interneuron migration in a ferret model of cortical dysplasia.Cereb Cortex. 2008 Jan;18(1):78-92. doi: 10.1093/cercor/bhm032. Epub 2007 Apr 18. Cereb Cortex. 2008. PMID: 17443019
-
KCC2 Manipulation Alters Features of Migrating Interneurons in Ferret Neocortex.Cereb Cortex. 2019 Dec 17;29(12):5072-5084. doi: 10.1093/cercor/bhz048. Cereb Cortex. 2019. PMID: 30953440
-
Influence of radial glia and Cajal-Retzius cells in neuronal migration.Results Probl Cell Differ. 2002;39:75-88. doi: 10.1007/978-3-540-46006-0_4. Results Probl Cell Differ. 2002. PMID: 12353469 Review.
-
Models of cortical malformation--Chemical and physical.J Neurosci Methods. 2016 Feb 15;260:62-72. doi: 10.1016/j.jneumeth.2015.03.034. Epub 2015 Apr 4. J Neurosci Methods. 2016. PMID: 25850077 Review.
Cited by
-
The Role of Redox Dysregulation in the Effects of Prenatal Stress on Embryonic Interneuron Migration.Cereb Cortex. 2019 Dec 17;29(12):5116-5130. doi: 10.1093/cercor/bhz052. Cereb Cortex. 2019. PMID: 30877797 Free PMC article.
-
Mapping arealisation of the visual cortex of non-primate species: lessons for development and evolution.Front Neural Circuits. 2014 Jul 4;8:79. doi: 10.3389/fncir.2014.00079. eCollection 2014. Front Neural Circuits. 2014. PMID: 25071460 Free PMC article. Review.
-
The Ferret as a Model System for Neocortex Development and Evolution.Front Cell Dev Biol. 2021 Apr 29;9:661759. doi: 10.3389/fcell.2021.661759. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33996819 Free PMC article. Review.
-
In vivo MRI Successfully Reveals the Malformation of Cortical Development in Infant Rats.Front Neurosci. 2020 May 20;14:510. doi: 10.3389/fnins.2020.00510. eCollection 2020. Front Neurosci. 2020. PMID: 32508585 Free PMC article.
-
Targeted disruption of layer 4 during development increases GABAA receptor neurotransmission in the neocortex.J Neurophysiol. 2014 Jan;111(2):323-35. doi: 10.1152/jn.00652.2012. Epub 2013 Oct 23. J Neurophysiol. 2014. PMID: 24155012 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

