Environmental stresses induce misfolded protein aggregation in plant cells in a microtubule-dependent manner
- PMID: 23574938
- PMCID: PMC3645715
- DOI: 10.3390/ijms14047771
Environmental stresses induce misfolded protein aggregation in plant cells in a microtubule-dependent manner
Abstract
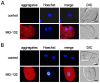
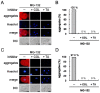
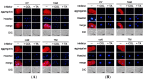
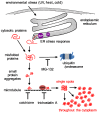
Misfolded protein aggregation in mammalian cells is one of the cellular responses to environmental stresses. However, the aggregation of misfolded proteins in plant cells exposed to environmental stresses is still poorly understood. Here, we report the misfolded protein aggregation in plant cells in response to environmental stresses, including ultraviolet (UV) radiation, heat stress and cold stress. Treatment of grape and tobacco cultured cells with MG-132, a proteasome inhibitor, induced misfolded protein aggregation. All of the environmental stresses examined induced the endoplasmic reticulum (ER) stress response in the cells. The cells under ER stress showed aggregation of misfolded proteins. The misfolded protein aggregation was completely inhibited by treatment of the cells with trichostatin A or colchicine, suggesting that the misfolded proteins might be aggregated in plant cells in a microtubule-dependent manner. Detected aggregates were initially observed immediately after exposure to the environmental stresses (1 min after UV radiation, 5 min after heat stress exposure, and 15 min after cold stress exposure). Based on these findings, we hypothesize that environmental stresses induce misfolded protein aggregation in plant cells in a microtubule-dependent manner.
Figures
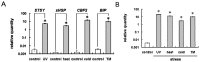


References
-
- Kamauchi S., Nakatani H., Nakano C., Urade R. Gene expression in response to endoplasmic reticulum stress in Arabidopsis thaliana. FEBS J. 2005;272:3461–3476. - PubMed
-
- Vidair A., Huang R.N., Doxsey S.J. Heat shock causes protein aggregation and reduced protein solubility at the centrosome and other cytoplasmic locations. Int. J. Hyperth. 1996;12:12681–12695. - PubMed
-
- Buchner J. Supervising the fold: Functional principles of molecular chaperones. FASEB J. 1996;10:10–19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

