Frataxin deficiency leads to reduced expression and impaired translocation of NF-E2-related factor (Nrf2) in cultured motor neurons
- PMID: 23574943
- PMCID: PMC3645720
- DOI: 10.3390/ijms14047853
Frataxin deficiency leads to reduced expression and impaired translocation of NF-E2-related factor (Nrf2) in cultured motor neurons
Abstract
Oxidative stress has been implicated in the pathogenesis of Friedreich's Ataxia (FRDA), a neurodegenerative disease caused by the decreased expression of frataxin, a mitochondrial protein responsible of iron homeostasis. Under conditions of oxidative stress, the activation of the transcription factor NF-E2-related factor (Nrf2) triggers the antioxidant cellular response by inducing antioxidant response element (ARE) driven genes. Increasing evidence supports a role for the Nrf2-ARE pathway in neurodegenerative diseases. In this study, we analyzed the expression and the distribution of Nrf2 in silenced neurons for frataxin gene. Decreased Nrf2 mRNA content and a defective activation after treatment with pro-oxidants have been evidenced in frataxin-silenced neurons by RT-PCR and confocal microscopy. The loss of Nrf2 in FRDA may greatly enhance the cellular susceptibility to oxidative stress and make FRDA neurons more vulnerable to injury. Our findings may help to focus on this promising target, especially in its emerging role in the neuroprotective response.
Figures
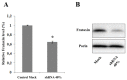
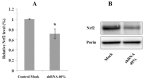



References
-
- Oppenheimer D., Esiri M. Disease of the Basal Ganglia, Cerebellum and Motor Neurons. In: Adams J.H., Corsellis J., Duchen L.W., editors. Greenfield’s Neuropathology. 5th ed. Arnold; London, UK: 1992. pp. 988–1054.
-
- Fogel B.L., Perlman S. Clinical features and molecular genetics of autosomal recessive cerebellar ataxias. Lancet Neurol. 2007;6:245–257. - PubMed
-
- Alper G., Narayanan V. Friedreich’s ataxia. Pediatr. Neurol. 2003;28:335–341. - PubMed
-
- Sakamato N., Chastain P.D., Parniewski P., Ohshima K., Pandolfo M., Griffith J.D., Wells R.D. Sticky DNA: Self-association properties of long GAA TTC repeats in RR Y triplex structures from Friedreich’s ataxia. Mol. Cell. 1999;3:465–475. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

