Temporal metatranscriptomic patterning in phototrophic Chloroflexi inhabiting a microbial mat in a geothermal spring
- PMID: 23575369
- PMCID: PMC3749495
- DOI: 10.1038/ismej.2013.52
Temporal metatranscriptomic patterning in phototrophic Chloroflexi inhabiting a microbial mat in a geothermal spring
Abstract
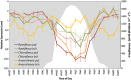
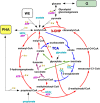
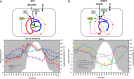
Filamentous anoxygenic phototrophs (FAPs) are abundant members of microbial mat communities inhabiting neutral and alkaline geothermal springs. Natural populations of FAPs related to Chloroflexus spp. and Roseiflexus spp. have been well characterized in Mushroom Spring, where they occur with unicellular cyanobacteria related to Synechococcus spp. strains A and B'. Metatranscriptomic sequencing was applied to the microbial community to determine how FAPs regulate their gene expression in response to fluctuating environmental conditions and resource availability over a diel period. Transcripts for genes involved in the biosynthesis of bacteriochlorophylls (BChls) and photosynthetic reaction centers were much more abundant at night. Both Roseiflexus spp. and Chloroflexus spp. expressed key genes involved in the 3-hydroxypropionate (3-OHP) carbon dioxide fixation bi-cycle during the day, when these FAPs have been thought to perform primarily photoheterotrophic and/or aerobic chemoorganotrophic metabolism. The expression of genes for the synthesis and degradation of storage polymers, including glycogen, polyhydroxyalkanoates and wax esters, suggests that FAPs produce and utilize these compounds at different times during the diel cycle. We summarize these results in a proposed conceptual model for temporal changes in central carbon metabolism and energy production of FAPs living in a natural environment. The model proposes that, at night, Chloroflexus spp. and Roseiflexus spp. synthesize BChl, components of the photosynthetic apparatus, polyhydroxyalkanoates and wax esters in concert with fermentation of glycogen. It further proposes that, in daytime, polyhydroxyalkanoates and wax esters are degraded and used as carbon and electron reserves to support photomixotrophy via the 3-OHP bi-cycle.
Figures
References
-
- Allewalt JP, Bateson MM, Revsbech NP, Slack K, Ward DM. Effect of temperature and light on growth of and photosynthesis by Synechococcus isolates typical of those predominating in the Octopus Spring microbial mat community of Yellowstone National Park. Appl Environ Microbiol. 2006;72:544–550. - PMC - PubMed
-
- Bassham JA, Kirk M. The effect of oxygen on the reduction of CO2 to glycolic acid and other products during photosynthesis by Chlorella. Biochem Biophys Res Commun. 1962;9:376–380. - PubMed
-
- Bhaya D, Grossman AR, Steunou A-S, Khuri N, Cohan FM, Hamamura N, et al. Population level functional diversity in a microbial community revealed by comparative genomic and metagenomic analyses. ISME J. 2007;1:703–713. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

