Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex
- PMID: 23575669
- PMCID: PMC3625970
- DOI: 10.1038/ncomms2647
Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex
Abstract
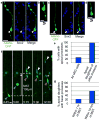
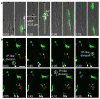
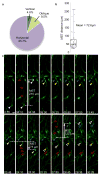
The human neocortex is increased in size and complexity as compared with most other species. Neocortical expansion has recently been attributed to protracted neurogenesis by outer radial glial cells in the outer subventricular zone, a region present in humans but not in rodents. The mechanisms of human outer radial glial cell generation are unknown, but are proposed to involve division of ventricular radial glial cells; neural stem cells present in all developing mammals. Here we show that human ventricular radial glial cells produce outer radial glial cells and seed formation of the outer subventricular zone via horizontal divisions, which occur more frequently in humans than in rodents. We further find that outer radial glial cell mitotic behaviour is cell intrinsic, and that the basal fibre, inherited by outer radial glial cells after ventricular radial glial division, determines cleavage angle. Our results suggest that altered regulation of mitotic spindle orientation increased outer radial glial cell number, and ultimately neuronal number, during human brain evolution.
Figures



References
-
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. - PubMed
-
- Fietz SA, et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

