VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation
- PMID: 23575676
- PMCID: PMC3644080
- DOI: 10.1038/ncomms2683
VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation
Abstract
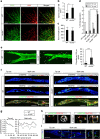
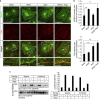
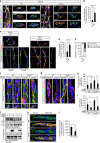
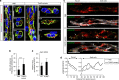
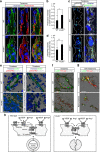
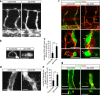
Vascular endothelial growth factor (VEGF) guides the path of new vessel sprouts by inducing VEGF receptor-2 activity in the sprout tip. In the stalk cells of the sprout, VEGF receptor-2 activity is downregulated. Here, we show that VEGF receptor-2 in stalk cells is dephosphorylated by the endothelium-specific vascular endothelial-phosphotyrosine phosphatase (VE-PTP). VE-PTP acts on VEGF receptor-2 located in endothelial junctions indirectly, via the Angiopoietin-1 receptor Tie2. VE-PTP inactivation in mouse embryoid bodies leads to excess VEGF receptor-2 activity in stalk cells, increased tyrosine phosphorylation of VE-cadherin and loss of cell polarity and lumen formation. Vessels in ve-ptp(-/-) teratomas also show increased VEGF receptor-2 activity and loss of endothelial polarization. Moreover, the zebrafish VE-PTP orthologue ptp-rb is essential for polarization and lumen formation in intersomitic vessels. We conclude that the role of Tie2 in maintenance of vascular quiescence involves VE-PTP-dependent dephosphorylation of VEGF receptor-2, and that VEGF receptor-2 activity regulates VE-cadherin tyrosine phosphorylation, endothelial cell polarity and lumen formation.
Figures


References
-
- Koch S., Tugues S., Li X., Gualandi L. & Claesson-Welsh L.. Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 437, 169–183 (2011) . - PubMed
-
- Carmeliet P. et al.. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 (1996) . - PubMed
-
- Ferrara N. et al.. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442 (1996) . - PubMed
-
- Shalaby F. et al.. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376, 62–66 (1995) . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

