Angiotensin II modulates salty and sweet taste sensitivities
- PMID: 23575826
- PMCID: PMC6619077
- DOI: 10.1523/JNEUROSCI.5599-12.2013
Angiotensin II modulates salty and sweet taste sensitivities
Abstract
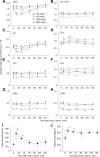
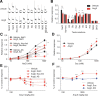
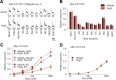
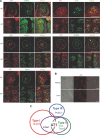
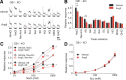
Understanding the mechanisms underlying gustatory detection of dietary sodium is important for the prevention and treatment of hypertension. Here, we show that Angiotensin II (AngII), a major mediator of body fluid and sodium homeostasis, modulates salty and sweet taste sensitivities, and that this modulation critically influences ingestive behaviors in mice. Gustatory nerve recording demonstrated that AngII suppressed amiloride-sensitive taste responses to NaCl. Surprisingly, AngII also enhanced nerve responses to sweeteners, but had no effect on responses to KCl, sour, bitter, or umami tastants. These effects of AngII on nerve responses were blocked by the angiotensin II type 1 receptor (AT1) antagonist CV11974. In behavioral tests, CV11974 treatment reduced the stimulated high licking rate to NaCl and sweeteners in water-restricted mice with elevated plasma AngII levels. In taste cells AT1 proteins were coexpressed with αENaC (epithelial sodium channel α-subunit, an amiloride-sensitive salt taste receptor) or T1r3 (a sweet taste receptor component). These results suggest that the taste organ is a peripheral target of AngII. The specific reduction of amiloride-sensitive salt taste sensitivity by AngII may contribute to increased sodium intake. Furthermore, AngII may contribute to increased energy intake by enhancing sweet responses. The linkage between salty and sweet preferences via AngII signaling may optimize sodium and calorie intakes.
Figures


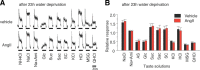
References
-
- Abaffy T, Trubey KR, Chaudhari N. Adenylyl cyclase expression and modulation of cAMP in rat taste cells. Am J Physiol Cell Physiol. 2003;284:C1420–C1428. - PubMed
-
- Billet S, Bardin S, Verp S, Baudrie V, Michaud A, Conchon S, Muffat-Joly M, Escoubet B, Souil E, Hamard G, Bernstein KE, Gasc JM, Elghozi JL, Corvol P, Clauser E. Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J Clin Invest. 2007;117:1914–1925. doi: 10.1172/JCI28764. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
