Mapping brain metabolic connectivity in awake rats with μPET and optogenetic stimulation
- PMID: 23575833
- PMCID: PMC3666931
- DOI: 10.1523/JNEUROSCI.4997-12.2013
Mapping brain metabolic connectivity in awake rats with μPET and optogenetic stimulation
Abstract
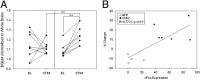
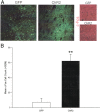
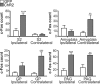
Positron emission tomography (PET) with [(18)F]2-fluoro-2-deoxy-D-glucose was used to measure changes in regional brain glucose metabolism (BGluM) in response to optogenetic stimulation (using the excitatory channelrhodopsin-2) of the nucleus accumbens (NAc) in awake rats. We demonstrated not only increases in BGluM that correlated with c-Fos expression in the region of stimulation, but also BGluM increases in the ipsilateral striatum, periaqueductal gray, and somatosensory cortex, and in contralateral amygdala, ventral pallidum, globus pallidus, and hippocampus, as well as decreases in BGluM in regions of the default mode network (retrosplenial cortex and cingulate gyrus) and secondary motor cortex. Additional exploration of c-Fos expression in regions found to be activated by PET results found corroborating evidence, with increased c-Fos expression in the ipsilateral somatosensory cortex, contralateral amygdala and globus pallidus, and bilateral periaqueductal gray. These findings are consistent with optogenetic excitation of the area of stimulation (NAc), as well as with stimulatory and inhibitory effects on downstream regions. They also confirm the utility of PET imaging to monitor connectivity in the awake rodent brain.
Figures

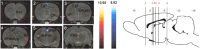
References
-
- Apostolova I, Block S, Buchert R, Osen B, Conradi M, Tabrizian S, Gensichen S, Schröder-Hartwig K, Fricke S, Rufer M, Weiss A, Hand I, Clausen M, Obrocki J. Effects of behavioral therapy or pharmacotherapy on brain glucose metabolism in subjects with obsessive-compulsive disorder as assessed by brain FDG PET. Psychiatry Res. 2010;184:105–116. doi: 10.1016/j.pscychresns.2010.08.012. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
