Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons
- PMID: 23575843
- PMCID: PMC3865539
- DOI: 10.1523/JNEUROSCI.0178-13.2013
Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons
Abstract
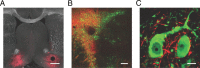
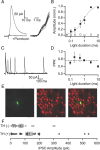
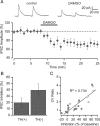
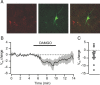
Activation of mu opioid receptors within the ventral tegmental area (VTA) can produce reward through the inhibition of GABAergic inputs. GABAergic neurons in the ventral pallidum (VP) provide a major input to VTA neurons. To determine the specific VTA neuronal targets of VP afferents and their sensitivity to mu opioid receptor agonists, we virally expressed channel rhodopsin (ChR2) in rat VP neurons and optogenetically activated their terminals in the VTA. Light activation of VP neuron terminals elicited GABAergic IPSCs in both dopamine (DA) and non-DA VTA neurons, and these IPSCs were inhibited by the mu opioid receptor agonist DAMGO. In addition, using a fluorescent retrograde marker to identify VTA-projecting VP neurons, we found them to be hyperpolarized by DAMGO. Both of these actions decrease GABAergic input onto VTA neurons, revealing two mechanisms by which endogenous or exogenous opioids can activate VTA neurons, including DA neurons.
Figures
References
-
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. - DOI - PMC - PubMed
-
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
