GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons
- PMID: 23575858
- PMCID: PMC3657840
- DOI: 10.1523/JNEUROSCI.0781-13.2013
GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons
Abstract
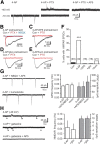
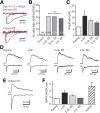
Neural activity enhances adult neurogenesis, enabling experience to influence the construction of new circuits. GABAA receptor-mediated depolarization of newborn neurons in the adult and developing brain promotes glutamatergic synaptic integration since chronic reduction of GABA depolarization impairs morphological maturation and formation of glutamatergic synapses. Here we demonstrate an acute role of GABA depolarization in glutamatergic synaptic integration. Using proopiomelanocortin enhanced-green fluorescent protein reporter mice, we identify a developmental stage when adult-generated neurons have glutamatergic synaptic transmission mediated solely by NMDA receptors (NMDARs), representing the initial silent synapses before AMPA receptor (AMPAR)-mediated functional transmission. We show that pairing synaptic stimulation with postsynaptic depolarization results in synapse unsilencing that requires NMDAR activation. GABA synaptic depolarization enables activation of NMDARs in the absence of AMPAR-mediated transmission and is required for synapse unsilencing induced by synaptic activity in vitro as well as a brief exposure to an enriched environment in vivo. The rapid appearance of AMPAR-mediated EPSCs and the lack of maturational changes show that GABA depolarization acutely allows NMDAR activation required for initial synapse unsilencing. Together, these results also reveal that adult-generated neurons in a critical period for survival use GABA signaling to rapidly initiate functional glutamate-mediated transmission in response to experience.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS047466/NS/NINDS NIH HHS/United States
- P30 NS057098/NS/NINDS NIH HHS/United States
- NS064025/NS/NINDS NIH HHS/United States
- NS065920/NS/NINDS NIH HHS/United States
- T32 NS061788/NS/NINDS NIH HHS/United States
- P30-HD38985/HD/NICHD NIH HHS/United States
- R01 NS064025/NS/NINDS NIH HHS/United States
- R01 NS065920/NS/NINDS NIH HHS/United States
- NS061788/NS/NINDS NIH HHS/United States
- P30-NS47466/NS/NINDS NIH HHS/United States
- P30-NS57098/NS/NINDS NIH HHS/United States
- P30 HD038985/HD/NICHD NIH HHS/United States
- NS078887/NS/NINDS NIH HHS/United States
- R56 NS064025/NS/NINDS NIH HHS/United States
- F31 NS078887/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
