Role of SAP97 protein in the regulation of corticotropin-releasing factor receptor 1 endocytosis and extracellular signal-regulated kinase 1/2 signaling
- PMID: 23576434
- PMCID: PMC3663523
- DOI: 10.1074/jbc.M113.473660
Role of SAP97 protein in the regulation of corticotropin-releasing factor receptor 1 endocytosis and extracellular signal-regulated kinase 1/2 signaling
Abstract
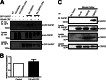
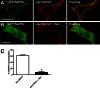
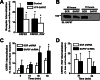
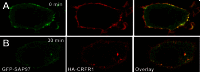
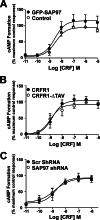
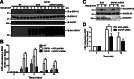
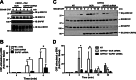
The corticotropin-releasing factor (CRF) receptor 1 (CRFR1) is a target for the treatment of psychiatric diseases such as depression, schizophrenia, anxiety disorder, and bipolar disorder. The carboxyl-terminal tail of the CRFR1 terminates in a PDZ-binding motif that provides a potential site for the interaction of PSD-95/Discs Large/Zona Occludens 1 (PDZ) domain-containing proteins. In this study, we found that CRFR1 interacts with synapse-associated protein 97 (SAP97; also known as DLG1) by co-immunoprecipitation in human embryonic 293 (HEK 293) cells and cortical brain lysates and that this interaction is dependent upon an intact PDZ-binding motif at the end of the CRFR1 carboxyl-terminal tail. Similarly, we demonstrated that SAP97 is recruited to the plasma membrane in HEK 293 cells expressing CRFR1 and that mutation of the CRFR1 PDZ-binding motif results in the redistribution of SAP97 into the cytoplasm. Overexpression of SAP97 antagonized agonist-stimulated CRFR1 internalization, whereas single hairpin (shRNA) knockdown of endogenous SAP97 in HEK 293 cells resulted in increased agonist-stimulated CRFR1 endocytosis. CRFR1 was internalized as a complex with SAP97 resulting in the redistribution of SAP97 to endocytic vesicles. Overexpression or shRNA knockdown of SAP97 did not significantly affect CRFR1-mediated cAMP formation, but SAP97 knockdown did significantly attenuate CRFR1-stimulated ERK1/2 phosphorylation in a PDZ interaction-independent manner. Taken together, our studies show that SAP97 interactions with CRFR1 attenuate CRFR1 endocytosis and that SAP97 is involved in coupling G protein-coupled receptors to the activation of the ERK1/2 signaling pathway.
Keywords: Cyclic AMP (cAMP); ERK; Endocytosis; G Protein-coupled Receptors (GPCR); Receptor Endocytosis; Signal Transduction.
Figures

Similar articles
-
Role of SAP97 in the regulation of 5-HT2AR endocytosis and signaling.Mol Pharmacol. 2014 Sep;86(3):275-83. doi: 10.1124/mol.114.093476. Epub 2014 Jul 2. Mol Pharmacol. 2014. PMID: 24989932
-
PDZK1/NHERF3 differentially regulates corticotropin-releasing factor receptor 1 and serotonin 2A receptor signaling and endocytosis.Cell Signal. 2015 Mar;27(3):519-31. doi: 10.1016/j.cellsig.2014.12.019. Epub 2015 Jan 3. Cell Signal. 2015. PMID: 25562428
-
PSD-95 regulates CRFR1 localization, trafficking and β-arrestin2 recruitment.Cell Signal. 2016 May;28(5):531-540. doi: 10.1016/j.cellsig.2016.02.013. Epub 2016 Feb 17. Cell Signal. 2016. PMID: 26898829
-
PDZ Protein Regulation of G Protein-Coupled Receptor Trafficking and Signaling Pathways.Mol Pharmacol. 2015 Oct;88(4):624-39. doi: 10.1124/mol.115.098509. Epub 2015 Mar 25. Mol Pharmacol. 2015. PMID: 25808930 Review.
-
Involvement of SAP97 anchored multiprotein complexes in regulating cardiorenal signaling and trafficking networks.Biochem Pharmacol. 2023 Feb;208:115406. doi: 10.1016/j.bcp.2022.115406. Epub 2022 Dec 31. Biochem Pharmacol. 2023. PMID: 36596415 Review.
Cited by
-
Resequencing and Association Analysis of Six PSD-95-Related Genes as Possible Susceptibility Genes for Schizophrenia and Autism Spectrum Disorders.Sci Rep. 2016 Jun 7;6:27491. doi: 10.1038/srep27491. Sci Rep. 2016. PMID: 27271353 Free PMC article.
-
Sex Differences in the Rat Hippocampal Opioid System After Oxycodone Conditioned Place Preference.Neuroscience. 2018 Nov 21;393:236-257. doi: 10.1016/j.neuroscience.2018.10.002. Epub 2018 Oct 11. Neuroscience. 2018. PMID: 30316908 Free PMC article.
-
MAGI Proteins Regulate the Trafficking and Signaling of Corticotropin-Releasing Factor Receptor 1 via a Compensatory Mechanism.J Mol Signal. 2016 Nov 28;11:5. doi: 10.5334/1750-2187-11-5. J Mol Signal. 2016. PMID: 31051013 Free PMC article.
-
Suppression of piriform cortex activity in rat by corticotropin-releasing factor 1 and serotonin 2A/C receptors.Front Cell Neurosci. 2015 May 28;9:200. doi: 10.3389/fncel.2015.00200. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26074770 Free PMC article.
-
G protein-coupled receptors: what a difference a 'partner' makes.Int J Mol Sci. 2014 Jan 16;15(1):1112-42. doi: 10.3390/ijms15011112. Int J Mol Sci. 2014. PMID: 24441568 Free PMC article. Review.
References
-
- Vale W., Spiess J., Rivier C., Rivier J. (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213, 1394–1397 - PubMed
-
- Leonard B. E. (2005) The HPA and immune axes in stress: the involvement of the serotonergic system. Eur. Psychiatry 20, S302–S306 - PubMed
-
- Chalmers D. T., Lovenberg T. W., Grigoriadis D. E., Behan D. P., De Souza E. B. (1996) Corticotrophin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol. Sci. 17, 166–172 - PubMed
-
- Dautzenberg F. M., Hauger R. L. (2002) The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol. Sci. 23, 71–77 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

