Rotavirus-encoded nonstructural protein 1 modulates cellular apoptotic machinery by targeting tumor suppressor protein p53
- PMID: 23576507
- PMCID: PMC3676122
- DOI: 10.1128/JVI.00734-13
Rotavirus-encoded nonstructural protein 1 modulates cellular apoptotic machinery by targeting tumor suppressor protein p53
Erratum in
-
Correction for Bhowmick et al., "Rotavirus-Encoded Nonstructural Protein 1 Modulates Cellular Apoptotic Machinery by Targeting Tumor Suppressor Protein p53".J Virol. 2021 Feb 24;95(6):e02302-20. doi: 10.1128/JVI.02302-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33627517 Free PMC article. No abstract available.
Abstract
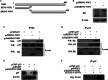
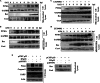
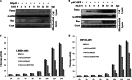
p53, a member of the innate immune system, is triggered under stress to induce cell growth arrest and apoptosis. Thus, p53 is an important target for viruses, as efficient infection depends on modulation of the host apoptotic machinery. This study focuses on how rotaviruses manipulate intricate p53 signaling for their advantage. Analysis of p53 expression revealed degradation of p53 during initial stages of rotavirus infection. However, in nonstructural protein-1 (NSP1) mutant strain A5-16, p53 degradation was not observed, suggesting a role of NSP1 in this process. This function of NSP1 was independent of its interferon or phosphatidylinositol 3-kinase (PI3K)/AKT modulation activity since p53 degradation was observed in Vero cells as well as in the presence of PI3K inhibitor. p53 transcript levels remained the same in SA11-infected cells (at 2 to 14 h postinfection), but p53 protein was stabilized only in the presence of MG132, suggesting a posttranslational process. NSP1 interacted with the DNA binding domain of p53, resulting in ubiquitination and proteasomal degradation of p53. Degradation of p53 during initial stages of infection inhibited apoptosis, as the proapoptotic genes PUMA and Bax were downregulated. During late viral infection, when progeny dissemination is the main objective, the NSP1-p53 interaction was diminished, resulting in restoration of the p53 level, with initiation of proapoptotic signaling ensuing. Overall results highlight the multiple strategies evolved by NSP1 to combat the host immune response.
Figures





Similar articles
-
Rotavirus nonstructural protein 1 suppresses virus-induced cellular apoptosis to facilitate viral growth by activating the cell survival pathways during early stages of infection.J Virol. 2010 Jul;84(13):6834-45. doi: 10.1128/JVI.00225-10. Epub 2010 Apr 14. J Virol. 2010. PMID: 20392855 Free PMC article.
-
Rotavirus NSP1 Subverts the Antiviral Oligoadenylate Synthetase-RNase L Pathway by Inducing RNase L Degradation.mBio. 2022 Dec 20;13(6):e0299522. doi: 10.1128/mbio.02995-22. Epub 2022 Nov 22. mBio. 2022. PMID: 36413023 Free PMC article.
-
Rotavirus NSP1 Requires Casein Kinase II-Mediated Phosphorylation for Hijacking of Cullin-RING Ligases.mBio. 2017 Aug 29;8(4):e01213-17. doi: 10.1128/mBio.01213-17. mBio. 2017. PMID: 28851847 Free PMC article.
-
The Rotavirus Interferon Antagonist NSP1: Many Targets, Many Questions.J Virol. 2016 May 12;90(11):5212-5215. doi: 10.1128/JVI.03068-15. Print 2016 Jun 1. J Virol. 2016. PMID: 27009959 Free PMC article. Review.
-
Rotavirus and reovirus modulation of the interferon response.J Interferon Cytokine Res. 2009 Sep;29(9):559-67. doi: 10.1089/jir.2009.0072. J Interferon Cytokine Res. 2009. PMID: 19694545 Free PMC article. Review.
Cited by
-
Treading a HOSTile path: Mapping the dynamic landscape of host cell-rotavirus interactions to explore novel host-directed curative dimensions.Virulence. 2021 Dec;12(1):1022-1062. doi: 10.1080/21505594.2021.1903198. Virulence. 2021. PMID: 33818275 Free PMC article. Review.
-
Silencing the alarms: Innate immune antagonism by rotavirus NSP1 and VP3.Virology. 2015 May;479-480:75-84. doi: 10.1016/j.virol.2015.01.006. Epub 2015 Feb 25. Virology. 2015. PMID: 25724417 Free PMC article. Review.
-
Rotavirus-Mediated Suppression of miRNA-192 Family and miRNA-181a Activates Wnt/β-Catenin Signaling Pathway: An In Vitro Study.Viruses. 2022 Mar 9;14(3):558. doi: 10.3390/v14030558. Viruses. 2022. PMID: 35336965 Free PMC article.
-
MAVS protein is attenuated by rotavirus nonstructural protein 1.PLoS One. 2014 Mar 18;9(3):e92126. doi: 10.1371/journal.pone.0092126. eCollection 2014. PLoS One. 2014. PMID: 24643253 Free PMC article.
-
The BM2 protein of influenza B virus interacts with p53 and inhibits its transcriptional and apoptotic activities.Mol Cell Biochem. 2015 May;403(1-2):187-97. doi: 10.1007/s11010-015-2349-7. Epub 2015 Feb 11. Mol Cell Biochem. 2015. PMID: 25670017
References
-
- Lane DP, Crawford LV. 1979. T antigen is bound to a host protein in SV40-transformed cells. Nature 278:261–263 - PubMed
-
- Linzer DI, Levine AJ. 1979. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 17:43–52 - PubMed
-
- Ryan KM. 2010. Cancer: viruses backup plan. Nature 466:1054–1055 - PubMed
-
- Vousden KH, Lane DP. 2007. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 8:275–283 - PubMed
-
- Vogelstein B, Lane D, Levine AJ. 2000. Surfing the p53 network. Nature 408:307–310 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

