Visualizing the beta interferon response in mice during infection with influenza A viruses expressing or lacking nonstructural protein 1
- PMID: 23576514
- PMCID: PMC3676098
- DOI: 10.1128/JVI.00283-13
Visualizing the beta interferon response in mice during infection with influenza A viruses expressing or lacking nonstructural protein 1
Abstract
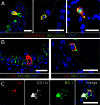
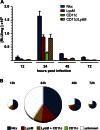
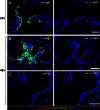
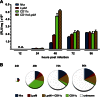
The innate host defense against influenza virus is largely dependent on the type I interferon (IFN) system. However, surprisingly little is known about the cellular source of IFN in the infected lung. To clarify this question, we employed a reporter mouse that contains the firefly luciferase gene in place of the IFN-β-coding region. IFN-β-producing cells were identified either by simultaneous immunostaining of lungs for luciferase and cellular markers or by generating conditional reporter mice that express luciferase exclusively in defined cell types. Two different strains of influenza A virus were employed that either do or do not code for nonstructural protein 1 (NS1), which strongly suppresses innate immune responses of infected cells. We found that epithelial cells and lung macrophages, which represent the prime host cells for influenza viruses, showed vigorous IFN-β responses which, however, were severely reduced and delayed if the infecting virus was able to produce NS1. Interestingly, CD11c(+) cell populations that were either expressing or lacking macrophage markers produced the bulk of IFN-β at 48 h after infection with wild-type influenza A virus. Our results demonstrate that the virus-encoded IFN-antagonistic factor NS1 disarms specifically epithelial cells and lung macrophages, which otherwise would serve as main mediators of the early response against infection by influenza virus.
Figures

References
-
- Genin P, Vaccaro A, Civas A. 2009. The role of differential expression of human interferon-α genes in antiviral immunity. Cytokine Growth Factor Rev. 20:283–295 - PubMed
-
- Erlandsson L, Blumenthal R, Eloranta ML, Engel H, Alm G, Weiss S, Leanderson T. 1998. Interferon-beta is required for interferon-alpha production in mouse fibroblasts. Curr. Biol. 8:223–226 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

