Complete protection against severe acute respiratory syndrome coronavirus-mediated lethal respiratory disease in aged mice by immunization with a mouse-adapted virus lacking E protein
- PMID: 23576515
- PMCID: PMC3676143
- DOI: 10.1128/JVI.00087-13
Complete protection against severe acute respiratory syndrome coronavirus-mediated lethal respiratory disease in aged mice by immunization with a mouse-adapted virus lacking E protein
Abstract
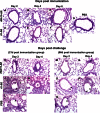
Zoonotic coronaviruses, including the one that caused severe acute respiratory syndrome (SARS), cause significant morbidity and mortality in humans. No specific therapy for any human coronavirus is available, making vaccine development critical for protection against these viruses. We previously showed that recombinant SARS coronavirus (SARS-CoV) (Urbani strain based) lacking envelope (E) protein expression (rU-ΔE) provided good but not perfect protection in young mice against challenge with virulent mouse-adapted SARS-CoV (MA15). To improve vaccine efficacy, we developed a second set of E-deleted vaccine candidates on an MA15 background (rMA15-ΔE). rMA15-ΔE is safe, causing no disease in 6-week-, 12-month-, or 18-month-old BALB/c mice. Immunization with this virus completely protected mice of three ages from lethal disease and effected more-rapid virus clearance. Compared to rU-ΔE, rMA15-ΔE immunization resulted in significantly greater neutralizing antibody and SARS-CoV-specific CD4 and CD8 T cell responses. After challenge, inflammatory cell infiltration, edema, and lung destruction were decreased in the lungs of rMA15-ΔE-immunized mice compared to those in rU-ΔE-immunized 12-month-old mice. Collectively, these results show that immunization with a species-adapted attenuated coronavirus lacking E protein expression is safe and provides optimal immunogenicity and long-term protection against challenge with lethal virus. This approach will be generally useful for development of vaccines protective against human coronaviruses as well as against coronaviruses that cause disease in domestic and companion animals.
Figures



References
-
- Lau SK, Li KS, Huang Y, Shek CT, Tse H, Wang M, Choi GK, Xu H, Lam CS, Guo R, Chan KH, Zheng BJ, Woo PC, Yuen KY. 2010. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J. Virol. 84:2808–2819 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

