DOG1 regulates growth and IGFBP5 in gastrointestinal stromal tumors
- PMID: 23576565
- PMCID: PMC3694361
- DOI: 10.1158/0008-5472.CAN-12-3839
DOG1 regulates growth and IGFBP5 in gastrointestinal stromal tumors
Abstract
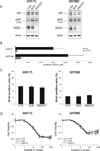
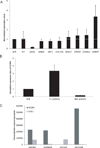
Gastrointestinal stromal tumors (GIST) are characterized by activating mutations of KIT or platelet-derived growth factor receptor α(PDGFRA), which can be therapeutically targeted by tyrosine kinase inhibitors (TKI) such as imatinib. Despite long-lasting responses, most patients eventually progress after TKI therapy. The calcium-dependent chloride channel DOG1 (ANO1/TMEM16A), which is strongly and specifically expressed in GIST, is used as a diagnostic marker to differentiate GIST from other sarcomas. Here, we report that loss of DOG1 expression occurs together with loss of KIT expression in a subset of GIST resistant to KIT inhibitors, and we illustrate the functional role of DOG1 in tumor growth, KIT expression, and imatinib response. Although DOG1 is a crucial regulator of chloride balance in GIST cells, we found that RNAi-mediated silencing or pharmacologic inhibition of DOG1 did not alter cell growth or KIT signaling in vitro. In contrast, DOG1 silencing delayed the growth of GIST xenografts in vivo. Expression profiling of explanted tumors after DOG1 blockade revealed a strong upregulation in the expression of insulin-like growth factor-binding protein 5 (IGFBP5), a potent antiangiogenic factor implicated in tumor suppression. Similar results were obtained after selection of imatinib-resistant DOG1- and KIT-negative cells derived from parental DOG1 and KIT-positive GIST cells, where a 5,000-fold increase in IGFBP5 mRNA transcripts were documented. In summary, our findings establish the oncogenic activity of DOG1 in GIST involving modulation of IGF/IGF receptor signaling in the tumor microenvironment through the antiangiogenic factor IGFBP5.
©2013 AACR.
Conflict of interest statement
Figures




References
-
- Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen C-J, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. - PubMed
-
- Hirota S. Gain-of-Function Mutations of c-kit in Human Gastrointestinal Stromal Tumors. Science. 1998;279:577–580. - PubMed
-
- Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, et al. KIT Activation Is a Ubiquitous Feature of Gastrointestinal Stromal Tumors. Cancer Res. 2001;61:8118–8121. - PubMed
-
- Blanke CD, Demetri GD, Von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. - PubMed
-
- Demetri G, von Mehren M, Blanke CD, Abbeele van den, Eisenberg BL, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

