NF-κB regulates radioresistance mediated by β1-integrin in three-dimensional culture of breast cancer cells
- PMID: 23576567
- PMCID: PMC3698967
- DOI: 10.1158/0008-5472.CAN-12-3537
NF-κB regulates radioresistance mediated by β1-integrin in three-dimensional culture of breast cancer cells
Abstract
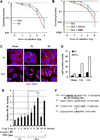
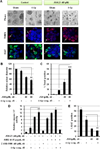
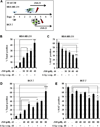
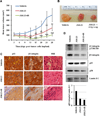
β1-integrin induction enhances breast cancer cell survival after exposure to ionizing radiation (IR), but the mechanisms of this effect remain unclear. Although NF-κB initiates prosurvival signaling pathways post-IR, the molecular function of NF-κB with other key elements in radioresistance, particularly with respect to extracellular matrix-induced signaling, is not known. We discovered a typical NF-κB-binding site in the β1-integrin promoter region, indicating a possible regulatory role for NF-κB. Using three-dimensional laminin-rich extracellular matrix (3D lrECM) culture, we show that NF-κB is required for β1-integrin transactivation in T4-2 breast cancer cells post-IR. Inhibition of NF-κB reduced clonogenic survival and induced apoptosis and cytostasis in formed tumor colonies. In addition, T4-2 tumors with inhibition of NF-κB activity exhibit decreased growth in athymic mice, which was further reduced by IR with downregulated β1-integrin expression. Direct interactions between β1-integrin and NF-κB p65 were induced in nonmalignant breast epithelial cells, but not in malignant cells, indicating context-specific regulation. As β1-integrin also activates NF-κB, our findings reveal a novel forward feedback pathway that could be targeted to enhance therapy.
©2013 AACR.
Conflict of interest statement
Conflict of interest: CP is a co-founder of Oncosynergy, ltd.
Figures

References
-
- Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8(7):545–554. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. - PubMed
-
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–1032. - PubMed
-
- Shaw LM. Integrin function in breast carcinoma progression. J Mammary Gland Biol Neoplasia. 1999;4(4):367–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

