Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients
- PMID: 23576625
- PMCID: PMC3716474
- DOI: 10.1212/WNL.0b013e3182904faa
Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients
Abstract
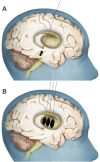
Objective: In an effort to account for deficiencies in axonal transport that limit the effectiveness of neurotrophic factors, this study tested the safety and feasibility, in moderately advanced Parkinson disease (PD), of bilaterally administering the gene therapy vector AAV2-neurturin (CERE-120) to the putamen plus substantia nigra (SN, a relatively small structure deep within the midbrain, in proximity to critical neuronal and vascular structures).
Methods: After planning and minimizing risks of stereotactically targeting the SN, an open-label, dose-escalation safety trial was initiated in 6 subjects with PD who received bilateral stereotactic injections of CERE-120 into the SN and putamen.
Results: Two-year safety data for all subjects suggest the procedures were well-tolerated, with no serious adverse events. All adverse events and complications were expected for patients with PD undergoing stereotactic brain surgery.
Conclusions: Bilateral stereotactic administration of CERE-120 to the SN plus putamen in PD is feasible and this evaluation provides initial empirical support that it is safe and well-tolerated.
Classification of evidence: This study provides Class IV evidence that bilateral neurturin gene delivery (CERE-120) to the SN plus putamen in patients with moderately advanced PD is feasible and safe.
Trial registration: ClinicalTrials.gov NCT00985517.
Figures
References
-
- Bartus RT, Baumann T, Brown L, Kruegel B, Ostrove JM, Herzog CD. Advancing neurotrophic factors as treatments for age-related neurodegenerative diseases: developing and demonstrating ‘clinical proof-of-concept’ for AAV-neurturin (CERE-120) in Parkinson’s disease. Neurobiol Aging 2013;34:35–61 - PubMed
-
- Marks WJ, Bartus RT, Siffert J, et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol 2010;9:1164–1172 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
