B cell response and hemagglutinin stalk-reactive antibody production in different age cohorts following 2009 H1N1 influenza virus vaccination
- PMID: 23576673
- PMCID: PMC3675965
- DOI: 10.1128/CVI.00735-12
B cell response and hemagglutinin stalk-reactive antibody production in different age cohorts following 2009 H1N1 influenza virus vaccination
Abstract
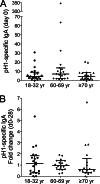
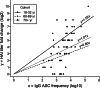
The 2009 pandemic H1N1 (pH1N1) influenza virus carried a swine-origin hemagglutinin (HA) that was closely related to the HAs of pre-1947 H1N1 viruses but highly divergent from the HAs of recently circulating H1N1 strains. Consequently, prior exposure to pH1N1-like viruses was mostly limited to individuals over the age of about 60 years. We related age and associated differences in immune history to the B cell response to an inactivated monovalent pH1N1 vaccine given intramuscularly to subjects in three age cohorts: 18 to 32 years, 60 to 69 years, and ≥70 years. The day 0 pH1N1-specific hemagglutination inhibition (HAI) and microneutralization (MN) titers were generally higher in the older cohorts, consistent with greater prevaccination exposure to pH1N1-like viruses. Most subjects in each cohort responded well to vaccination, with early formation of circulating virus-specific antibody (Ab)-secreting cells and ≥4-fold increases in HAI and MN titers. However, the response was strongest in the 18- to 32-year cohort. Circulating levels of HA stalk-reactive Abs were increased after vaccination, especially in the 18- to 32-year cohort, raising the possibility of elevated levels of cross-reactive neutralizing Abs. In the young cohort, an increase in MN activity against the seasonal influenza virus A/Brisbane/59/07 after vaccination was generally associated with an increase in the anti-Brisbane/59/07 HAI titer, suggesting an effect mediated primarily by HA head-reactive rather than stalk-reactive Abs. Our findings support recent proposals that immunization with a relatively novel HA favors the induction of Abs against conserved epitopes. They also emphasize the need to clarify how the level of circulating stalk-reactive Abs relates to resistance to influenza.
Figures




References
-
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201 - PMC - PubMed
-
- Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, Brammer TL, Cox NJ, Tumpey TM, Katz JM. 2009. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361:1945–1952 - PubMed
-
- Skowronski DM, Hottes TS, McElhaney JE, Janjua NZ, Sabaiduc S, Chan T, Gentleman B, Purych D, Gardy J, Patrick DM, Brunham RC, De Serres G, Petric M. 2011. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: higher antibody and lower cell-mediated immune responses with advanced age. J. Infect. Dis. 203:158–167 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

