Shaping organs by a wingless-int/Notch/nonmuscle myosin module which orients feather bud elongation
- PMID: 23576731
- PMCID: PMC3631647
- DOI: 10.1073/pnas.1219813110
Shaping organs by a wingless-int/Notch/nonmuscle myosin module which orients feather bud elongation
Abstract
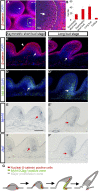
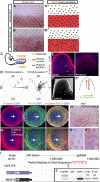
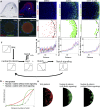
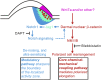
How organs are shaped to specific forms is a fundamental issue in developmental biology. To address this question, we used the repetitive, periodic pattern of feather morphogenesis on chicken skin as a model. Avian feathers within a single tract extend from dome-shaped primordia to thin conical structures with a common axis of orientation. From a systems biology perspective, the process is precise and robust. Using tissue transplantation assays, we demonstrate that a "zone of polarizing activity," localized in the posterior feather bud, is necessary and sufficient to mediate the directional elongation. This region contains a spatially well-defined nuclear β-catenin zone, which is induced by wingless-int (Wnt)7a protein diffusing in from posterior bud epithelium. Misexpressing nuclear β-catenin randomizes feather polarity. This dermal nuclear β-catenin zone, surrounded by Notch1 positive dermal cells, induces Jagged1. Inhibition of Notch signaling disrupts the spatial configuration of the nuclear β-catenin zone and leads to randomized feather polarity. Mathematical modeling predicts that lateral inhibition, mediated by Notch signaling, functions to reduce Wnt7a gradient variations and fluctuations to form the sharp boundary observed for the dermal β-catenin zone. This zone is also enriched for nonmuscle myosin IIB. Suppressing nonmuscle myosin IIB disrupts directional cell rearrangements and abolishes feather bud elongation. These data suggest that a unique molecular module involving chemical-mechanical coupling converts a pliable chemical gradient to a precise domain, ready for subsequent mechanical action, thus defining the position, boundary, and duration of localized morphogenetic activity that molds the shape of growing organs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Wnt-7a in feather morphogenesis: involvement of anterior-posterior asymmetry and proximal-distal elongation demonstrated with an in vitro reconstitution model.Development. 1999 Jun;126(12):2577-87. doi: 10.1242/dev.126.12.2577. Development. 1999. PMID: 10331970
-
Involvement of Hex in the initiation of feather morphogenesis.Int J Dev Biol. 2005;49(8):953-60. doi: 10.1387/ijdb.052079ao. Int J Dev Biol. 2005. PMID: 16281172
-
Shift of localized growth zones contributes to skin appendage morphogenesis: role of the Wnt/beta-catenin pathway.J Invest Dermatol. 2003 Jan;120(1):20-6. doi: 10.1046/j.1523-1747.2003.12008.x. J Invest Dermatol. 2003. PMID: 12535194 Free PMC article.
-
The making of a feather: homeoproteins, retinoids and adhesion molecules.Bioessays. 1993 Aug;15(8):513-21. doi: 10.1002/bies.950150804. Bioessays. 1993. PMID: 7907866 Review.
-
Signaling pathways regulating zebrafish lateral line development.Curr Biol. 2009 May 12;19(9):R381-6. doi: 10.1016/j.cub.2009.03.057. Curr Biol. 2009. PMID: 19439264 Review.
Cited by
-
Characterizing and controlling the inflammatory network during influenza A virus infection.Sci Rep. 2014 Jan 21;4:3799. doi: 10.1038/srep03799. Sci Rep. 2014. PMID: 24445954 Free PMC article.
-
Tissue patterning and cellular mechanics.J Cell Biol. 2015 Oct 26;211(2):219-31. doi: 10.1083/jcb.201506106. J Cell Biol. 2015. PMID: 26504164 Free PMC article. Review.
-
Development, regeneration, and evolution of feathers.Annu Rev Anim Biosci. 2015;3:169-95. doi: 10.1146/annurev-animal-022513-114127. Epub 2014 Nov 3. Annu Rev Anim Biosci. 2015. PMID: 25387232 Free PMC article. Review.
-
Transcriptomic analyses of regenerating adult feathers in chicken.BMC Genomics. 2015 Oct 6;16:756. doi: 10.1186/s12864-015-1966-6. BMC Genomics. 2015. PMID: 26445093 Free PMC article.
-
Differential network as an indicator of osteoporosis with network entropy.Exp Ther Med. 2018 Jul;16(1):328-332. doi: 10.3892/etm.2018.6169. Epub 2018 May 16. Exp Ther Med. 2018. PMID: 29896257 Free PMC article.
References
-
- Slack JM. Origin of stem cells in organogenesis. Science. 2008;322(5907):1498–1501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AR060306/AR/NIAMS NIH HHS/United States
- R01 GM067247/GM/NIGMS NIH HHS/United States
- P30DK048522/DK/NIDDK NIH HHS/United States
- P50GM76516/GM/NIGMS NIH HHS/United States
- GM67247/GM/NIGMS NIH HHS/United States
- AR47364/AR/NIAMS NIH HHS/United States
- R01 AR060306/AR/NIAMS NIH HHS/United States
- AR 42177/AR/NIAMS NIH HHS/United States
- AR60306/AR/NIAMS NIH HHS/United States
- R01 AR042177/AR/NIAMS NIH HHS/United States
- R01 AR047364/AR/NIAMS NIH HHS/United States
- P50 GM076516/GM/NIGMS NIH HHS/United States
- P30 DK048522/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

