Neural progenitors organize in small-world networks to promote cell proliferation
- PMID: 23576737
- PMCID: PMC3631687
- DOI: 10.1073/pnas.1220179110
Neural progenitors organize in small-world networks to promote cell proliferation
Abstract
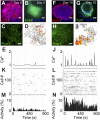
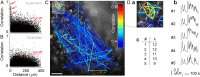
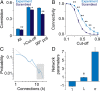
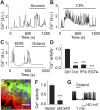
Coherent network activity among assemblies of interconnected cells is essential for diverse functions in the adult brain. However, cellular networks before formations of chemical synapses are poorly understood. Here, embryonic stem cell-derived neural progenitors were found to form networks exhibiting synchronous calcium ion (Ca(2+)) activity that stimulated cell proliferation. Immature neural cells established circuits that propagated electrical signals between neighboring cells, thereby activating voltage-gated Ca(2+) channels that triggered Ca(2+) oscillations. These network circuits were dependent on gap junctions, because blocking prevented electrotonic transmission both in vitro and in vivo. Inhibiting connexin 43 gap junctions abolished network activity, suppressed proliferation, and affected embryonic cortical layer formation. Cross-correlation analysis revealed highly correlated Ca(2+) activities in small-world networks that followed a scale-free topology. Graph theory predicts that such network designs are effective for biological systems. Taken together, these results demonstrate that immature cells in the developing brain organize in small-world networks that critically regulate neural progenitor proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Small-world networks of spontaneous Ca(2+) activity.Commun Integr Biol. 2013 Jul 1;6(4):e24788. doi: 10.4161/cib.24788. Epub 2013 May 10. Commun Integr Biol. 2013. PMID: 23986813 Free PMC article.
-
Adult neural precursor cells form connexin-dependent networks that improve their survival.Neuroreport. 2015 Oct 21;26(15):928-36. doi: 10.1097/WNR.0000000000000451. Neuroreport. 2015. PMID: 26351758
-
Network analysis of time-lapse microscopy recordings.Front Neural Circuits. 2014 Sep 17;8:111. doi: 10.3389/fncir.2014.00111. eCollection 2014. Front Neural Circuits. 2014. PMID: 25278844 Free PMC article.
-
Design principles of electrical synaptic plasticity.Neurosci Lett. 2019 Mar 16;695:4-11. doi: 10.1016/j.neulet.2017.09.003. Epub 2017 Sep 8. Neurosci Lett. 2019. PMID: 28893590 Free PMC article. Review.
-
Gap junctions: their importance for the dynamics of neural circuits.Mol Neurobiol. 2004 Dec;30(3):341-57. doi: 10.1385/MN:30:3:341. Mol Neurobiol. 2004. PMID: 15655256 Review.
Cited by
-
Improved estimation and interpretation of correlations in neural circuits.PLoS Comput Biol. 2015 Mar 31;11(3):e1004083. doi: 10.1371/journal.pcbi.1004083. eCollection 2015 Mar. PLoS Comput Biol. 2015. PMID: 25826696 Free PMC article.
-
Unprecedented cell-selection using ultra-quick freezing combined with aquaporin expression.PLoS One. 2014 Feb 18;9(2):e87644. doi: 10.1371/journal.pone.0087644. eCollection 2014. PLoS One. 2014. PMID: 24558371 Free PMC article.
-
Disrupted Cacna1c gene expression perturbs spontaneous Ca2+ activity causing abnormal brain development and increased anxiety.Proc Natl Acad Sci U S A. 2022 Feb 15;119(7):e2108768119. doi: 10.1073/pnas.2108768119. Proc Natl Acad Sci U S A. 2022. PMID: 35135875 Free PMC article.
-
From Isles of Königsberg to Islets of Langerhans: Examining the Function of the Endocrine Pancreas Through Network Science.Front Endocrinol (Lausanne). 2022 Jun 15;13:922640. doi: 10.3389/fendo.2022.922640. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35784543 Free PMC article. Review.
-
Secretagogin protects Pdx1 from proteasomal degradation to control a transcriptional program required for β cell specification.Mol Metab. 2018 Aug;14:108-120. doi: 10.1016/j.molmet.2018.05.019. Epub 2018 Jun 5. Mol Metab. 2018. PMID: 29910119 Free PMC article.
References
-
- McCulloch W, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys. 1943;5:115–133. - PubMed
-
- Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70(2):200–227. - PubMed
-
- Albert R, Barabasi AL. Statistical mechanics of complex networks. Rev Mod Phys. 2002;74(1):47–97.
-
- Feldt S, Bonifazi P, Cossart R. Dissecting functional connectivity of neuronal microcircuits: Experimental and theoretical insights. Trends Neurosci. 2011;34(5):225–236. - PubMed
-
- Barkai N, Leibler S. Robustness in simple biochemical networks. Nature. 1997;387(6636):913–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

