Characterization of mechanical and biochemical properties of developing embryonic tendon
- PMID: 23576745
- PMCID: PMC3631620
- DOI: 10.1073/pnas.1300135110
Characterization of mechanical and biochemical properties of developing embryonic tendon
Abstract
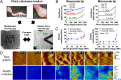
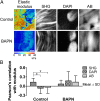
Tendons have uniquely high tensile strength, critical to their function to transfer force from muscle to bone. When injured, their innate healing response results in aberrant matrix organization and functional properties. Efforts to regenerate tendon are challenged by limited understanding of its normal development. Consequently, there are few known markers to assess tendon formation and parameters to design tissue engineering scaffolds. We profiled mechanical and biological properties of embryonic tendon and demonstrated functional properties of developing tendon are not wholly reflected by protein expression and tissue morphology. Using force volume-atomic force microscopy, we found that nano- and microscale tendon elastic moduli increase nonlinearly and become increasingly spatially heterogeneous during embryonic development. When we analyzed potential biochemical contributors to modulus, we found statistically significant but weak correlation between elastic modulus and collagen content, and no correlation with DNA or glycosaminoglycan content, indicating there are additional contributors to mechanical properties. To investigate collagen cross-linking as a potential contributor, we inhibited lysyl oxidase-mediated collagen cross-linking, which significantly reduced tendon elastic modulus without affecting collagen morphology or DNA, glycosaminoglycan, and collagen content. This suggests that lysyl oxidase-mediated cross-linking plays a significant role in the development of embryonic tendon functional properties and demonstrates that changes in cross-links alter mechanical properties without affecting matrix content and organization. Taken together, these data demonstrate the importance of functional markers to assess tendon development and provide a profile of tenogenic mechanical properties that may be implemented in tissue engineering scaffold design to mechanoregulate new tendon regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Butler DL, Awad HA. Perspectives on cell and collagen composites for tendon repair. Clin Orthop Relat Res. 1999;(367) Suppl:S324–S332. - PubMed
-
- Woo SL, Abramowitch SD, Kilger R, Liang R. Biomechanics of knee ligaments: Injury, healing, and repair. J Biomech. 2006;39(1):1–20. - PubMed
-
- AAOS . United States Bone and Joint Decade: The Burden of Musculoskeletal Diseases in the United States. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
-
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125(20):4019–4032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

