Memory reconsolidation and its maintenance depend on L-voltage-dependent calcium channels and CaMKII functions regulating protein turnover in the hippocampus
- PMID: 23576750
- PMCID: PMC3631664
- DOI: 10.1073/pnas.1302356110
Memory reconsolidation and its maintenance depend on L-voltage-dependent calcium channels and CaMKII functions regulating protein turnover in the hippocampus
Abstract
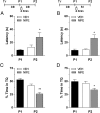
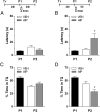
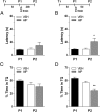
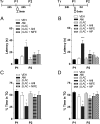
Immediate postretrieval bilateral blockade of long-acting voltage-dependent calcium channels (L-VDCCs), but not of glutamatergic NMDA receptors, in the dorsal CA1 region of the hippocampus hinders retention of long-term spatial memory in the Morris water maze. Immediate postretrieval bilateral inhibition of calcium/calmodulin-dependent protein kinase (CaMK) II in dorsal CA1 does not affect retention of this task 24 h later but does hinder it 5 d later. These two distinct amnesic effects are abolished if protein degradation by proteasomes is inhibited concomitantly. These results indicate that spatial memory reconsolidation depends on the functionality of L-VDCC in dorsal CA1, that maintenance of subsequent reconsolidated memory trace depends on CaMKII, and these results also suggest that the role played by both L-VDCC and CaMKII is to promote the retrieval-dependent, synaptically localized enhancement of protein synthesis necessary to counteract a retrieval-dependent, synaptic-localized enhancement of protein degradation, which has been described as underlying the characteristic labilization of the memory trace triggered by retrieval. Thus, conceivably, L-VDCC and CaMKII would enhance activity-dependent localized protein renewal, which may account for the improvement of the long-term efficiency of the synapses responsible for the maintenance of reactivated long-term spatial memory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The role of calcium-calmodulin-dependent protein kinase II in modulation of spatial memory in morphine sensitized rats.Behav Brain Res. 2019 Feb 1;359:298-303. doi: 10.1016/j.bbr.2018.11.010. Epub 2018 Nov 11. Behav Brain Res. 2019. PMID: 30428335
-
Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat.Learn Mem. 2000 Sep-Oct;7(5):333-40. doi: 10.1101/lm.34600. Learn Mem. 2000. PMID: 11040265 Free PMC article.
-
Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats.Peptides. 2006 Nov;27(11):2738-49. doi: 10.1016/j.peptides.2006.07.001. Epub 2006 Aug 17. Peptides. 2006. PMID: 16914228
-
Mechanisms of CaMKII action in long-term potentiation.Nat Rev Neurosci. 2012 Feb 15;13(3):169-82. doi: 10.1038/nrn3192. Nat Rev Neurosci. 2012. PMID: 22334212 Free PMC article. Review.
-
The molecular basis of CaMKII function in synaptic and behavioural memory.Nat Rev Neurosci. 2002 Mar;3(3):175-90. doi: 10.1038/nrn753. Nat Rev Neurosci. 2002. PMID: 11994750 Review.
Cited by
-
Regulation of seizure-induced MeCP2 Ser421 phosphorylation in the developing brain.Neurobiol Dis. 2018 Aug;116:120-130. doi: 10.1016/j.nbd.2018.05.001. Epub 2018 May 5. Neurobiol Dis. 2018. PMID: 29738885 Free PMC article.
-
Prelimbic proBDNF Facilitates Retrieval-Dependent Fear Memory Destabilization by Regulation of Synaptic and Neural Functions in Juvenile Rats.Mol Neurobiol. 2022 Jul;59(7):4179-4196. doi: 10.1007/s12035-022-02849-9. Epub 2022 Apr 30. Mol Neurobiol. 2022. PMID: 35501631
-
Memory destabilization during reconsolidation: a consequence of homeostatic plasticity?Learn Mem. 2021 Sep 15;28(10):371-389. doi: 10.1101/lm.053418.121. Print 2021 Oct. Learn Mem. 2021. PMID: 34526382 Free PMC article.
-
A Model of Synaptic Reconsolidation.Front Neurosci. 2016 May 18;10:206. doi: 10.3389/fnins.2016.00206. eCollection 2016. Front Neurosci. 2016. PMID: 27242410 Free PMC article.
-
Persistent long-term facilitation at an identified synapse becomes labile with activation of short-term heterosynaptic plasticity.J Neurosci. 2014 Apr 2;34(14):4776-85. doi: 10.1523/JNEUROSCI.0098-14.2014. J Neurosci. 2014. PMID: 24695698 Free PMC article.
References
-
- Cahill L, McGaugh JL. A novel demonstration of enhanced memory associated with emotional arousal. Conscious Cogn. 1995;4(4):410–421. - PubMed
-
- Cahill L, McGaugh JL. Modulation of memory storage. Curr Opin Neurobiol. 1996;6(2):237–242. - PubMed
-
- Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36(3):521–525. - PubMed
-
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. - PubMed
-
- Wang S-H, Morris RGM. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol. 2010;61:49–79, C1–C4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

