Modifying lignin to improve bioenergy feedstocks: strengthening the barrier against pathogens?
- PMID: 23577013
- PMCID: PMC3617363
- DOI: 10.3389/fpls.2013.00070
Modifying lignin to improve bioenergy feedstocks: strengthening the barrier against pathogens?
Abstract
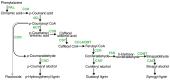
Lignin is a ubiquitous polymer present in cell walls of all vascular plants, where it rigidifies and strengthens the cell wall structure through covalent cross-linkages to cell wall polysaccharides. The presence of lignin makes the cell wall recalcitrant to conversion into fermentable sugars for bioenergy uses. Therefore, reducing lignin content and modifying its linkages have become major targets for bioenergy feedstock development through either biotechnology or traditional plant breeding. In addition, lignin synthesis has long been implicated as an important plant defense mechanism against pathogens, because lignin synthesis is often induced at the site of pathogen attack. This article explores the impact of lignin modifications on the susceptibility of a range of plant species to their associated pathogens, and the implications for development of feedstocks for the second-generation biofuels industry. Surprisingly, there are some instances where plants modified in lignin synthesis may display increased resistance to associated pathogens, which is explored in this article.
Keywords: CAD; COMT; brown midrib; lignin; monolignol pathway; plant pathogens.
Figures
Similar articles
-
Modifying crops to increase cell wall digestibility.Plant Sci. 2012 Apr;185-186:65-77. doi: 10.1016/j.plantsci.2011.10.014. Epub 2011 Oct 25. Plant Sci. 2012. PMID: 22325867 Review.
-
Altered lignin biosynthesis using biotechnology to improve lignocellulosic biofuel feedstocks.Plant Biotechnol J. 2014 Dec;12(9):1163-73. doi: 10.1111/pbi.12225. Epub 2014 Jul 22. Plant Biotechnol J. 2014. PMID: 25051990 Review.
-
Plant biotechnology for lignocellulosic biofuel production.Plant Biotechnol J. 2014 Dec;12(9):1174-92. doi: 10.1111/pbi.12273. Epub 2014 Oct 20. Plant Biotechnol J. 2014. PMID: 25330253 Review.
-
Expression of cell wall related genes in basal and ear internodes of silking brown-midrib-3, caffeic acid O-methyltransferase (COMT) down-regulated, and normal maize plants.BMC Plant Biol. 2008 Jun 26;8:71. doi: 10.1186/1471-2229-8-71. BMC Plant Biol. 2008. PMID: 18582385 Free PMC article.
-
Trends in lignin modification: a comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity.Phytochemistry. 2002 Oct;61(3):221-94. doi: 10.1016/s0031-9422(02)00211-x. Phytochemistry. 2002. PMID: 12359514 Review.
Cited by
-
Host Cell Wall Damage during Pathogen Infection: Mechanisms of Perception and Role in Plant-Pathogen Interactions.Plants (Basel). 2021 Feb 19;10(2):399. doi: 10.3390/plants10020399. Plants (Basel). 2021. PMID: 33669710 Free PMC article. Review.
-
Transciptome profiling at early infection of Elaeis guineensis by Ganoderma boninense provides novel insights on fungal transition from biotrophic to necrotrophic phase.BMC Plant Biol. 2018 Dec 29;18(1):377. doi: 10.1186/s12870-018-1594-9. BMC Plant Biol. 2018. PMID: 30594134 Free PMC article.
-
Plant Cell Wall Changes in Common Wheat Roots as a Result of Their Interaction with Beneficial Fungi of Trichoderma.Cells. 2020 Oct 19;9(10):2319. doi: 10.3390/cells9102319. Cells. 2020. PMID: 33086614 Free PMC article.
-
Towards uncovering the roles of switchgrass peroxidases in plant processes.Front Plant Sci. 2013 Jun 19;4:202. doi: 10.3389/fpls.2013.00202. eCollection 2013. Front Plant Sci. 2013. PMID: 23802005 Free PMC article.
-
Phenylpropanoid Biosynthesis Gene Expression Precedes Lignin Accumulation During Shoot Development in Lowland and Upland Switchgrass Genotypes.Front Plant Sci. 2021 Aug 9;12:640930. doi: 10.3389/fpls.2021.640930. eCollection 2021. Front Plant Sci. 2021. PMID: 34434200 Free PMC article.
References
-
- Ahonsi M. O., Ames K. A., Gray M. E., Bradley C. A. (2013). Biomass reducing potential and prospective fungicide control of a new leaf blight of Miscanthus × giganteus caused by Leptosphaerulina chartarum. Bioenergy Res. 10.1007/s12155-012-9293-0 - DOI
-
- Baayen R. P., Ouellette G. B., Rioux D. (1996). Compartmentalization of decay in carnations resistant to Fusarium oxysporum f. sp. dianthi. Phytopathology 86 1018–1031
-
- Beccari G., Covarelli L., Balmas V., Tosi L. (2010). First report of Miscanthusgiganteus rhizome rot caused by Fusarium avenaceum, Fusarium oxysporum and Mucor hiemalis. Australas. Plant Dis. Notes 5 28–29
-
- Beekrum S., Govinden R., Padayachee T., Odhav B. (2003). Naturally occurring phenols: a detoxification strategy for fumonisin B1. Food Addit. Contam. 20 490–493 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

