An update on the ocular phenotype in patients with pseudoxanthoma elasticum
- PMID: 23577018
- PMCID: PMC3617449
- DOI: 10.3389/fgene.2013.00014
An update on the ocular phenotype in patients with pseudoxanthoma elasticum
Abstract
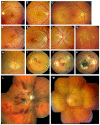
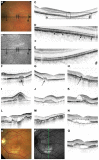
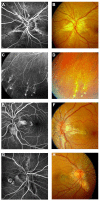
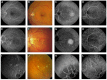
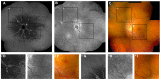
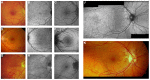
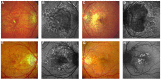
Pseudoxanthoma elasticum (PXE) is an inherited multi-system disorder characterized by ectopic mineralization and fragmentation of elastic fibers in the skin, the elastic laminae of blood vessels and Bruch's membrane in the eye. Biallelic mutations in the ATP-binding cassette (ABC) transporter gene ABCC6 on chromosome 16 are responsible for the disease. The pathophysiology is incompletely understood. However, there is consent that a metabolic alteration leads to dysfunction in extracellular calcium homeostasis and subsequent calcification of connective tissues rich in elastic fibers. This review summarizes and aims at explaining the variety of phenotypic ocular findings in patients with PXE. Specialized imaging techniques including white light fundus photography, blue light autofluorescence, near-infrared confocal reflectance imaging, high resolution optical coherence tomography, fluorescein and indocyanine green (ICG) angiography have revealed characteristic lesions at the ocular fundus of PXE patients. These include the classic signs of angioid streaks, peau d'orange, comet lesions, and choroidal neovascularizations (CNVs), but also the more recently recognized features such as chorioretinal atrophy, subretinal fluid independent from CNV, pattern dystrophy-like changes, debris accumulation under the retinal pigment epithelium, reticular drusen and a decreased fluorescence on late phase ICG angiography.
Keywords: Bruch’s membrane; angioid streaks; choroidal neovascularization; pseudoxanthoma elasticum; retina.
Figures
References
-
- Aessopos A., Farmakis D., Loukopoulos D. (2002). Elastic tissue abnormalities resembling pseudoxanthoma elasticum in beta thalassemia and the sickling syndromes. Blood. 99 30–35 - PubMed
-
- Agarwal A., Patel P., Adkins T., Gass J. D. (2005). Spectrum of pattern dystrophy in pseudoxanthoma elasticum. Arch. Ophthalmol. 123 923–928 - PubMed
-
- Audo I., Vanakker O. M., Smith A., Leroy B. P., Robson A. G., Jenkins S. A., et al. (2007). Pseudoxanthoma elasticum with generalized retinal dysfunction, a common finding? Invest. Ophthalmol. Vis. Sci. 48 4250–4256 - PubMed
-
- Baillif-Gostoli S., Quaranta-El Maftouhi M., Mauget-Faysse M. (2010). Polypoidal choroidal vasculopathy in a patient with angioid streaks secondary to pseudoxanthoma elasticum. Graefes Arch. Clin. Exp. Ophthalmol. 248 1845–1848 - PubMed
-
- Bergen A. A., Plomp A. S., Schuurman E. J., Terry S., Breuning M., Dauwerse H., et al. (2000). Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat. Genet. 25 228–231 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

