Epigenetic modification of the Epstein-Barr virus BZLF1 promoter regulates viral reactivation from latency
- PMID: 23577022
- PMCID: PMC3620531
- DOI: 10.3389/fgene.2013.00053
Epigenetic modification of the Epstein-Barr virus BZLF1 promoter regulates viral reactivation from latency
Abstract
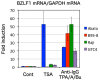
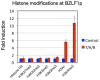
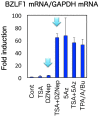
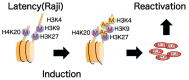
The Epstein-Barr virus (EBV) is an oncogenic human gamma-herpesvirus that predominantly establishes latent infection in B lymphocytes. Viral genomes exist as extrachromosomal episomes with a nucleosomal structure. Maintenance of virus latency or execution of reactivation is controlled by the expression of BZLF1, a viral immediate-early gene product, tightly controlled at the transcriptional level. In this article, we review how BZLF1 transcription is controlled, in other words how virus reactivation is regulated, especially in terms of epigenetics. We recently found that histone H3 lysine 27 trimethylation (H3K27me3) and H4K20me3 markers are crucial for suppression of BZLF1 in latent Raji cells. In addition, H3K9me2/3, heterochromatin protein 1, and H2A ubiquitination are associated with latency, whereas positive markers, such as higher histone acetylation and H3K4me3, are concomitant with reactivation. Since lytic replication eventually causes cell cycle arrest and cell death, development of oncolytic therapy for EBV-positive cancers is conceivable using epigenetic disruptors. In addition, we note the difficulties in analyzing roles of epigenetics in EBV, including issues like cell type dependence and virus copy numbers.
Keywords: BZLF1 gene; Epstein–Barr virus; epigenetics; latency; reactivation.
Figures
Similar articles
-
Epigenetic histone modification of Epstein-Barr virus BZLF1 promoter during latency and reactivation in Raji cells.J Virol. 2012 May;86(9):4752-61. doi: 10.1128/JVI.06768-11. Epub 2012 Feb 22. J Virol. 2012. PMID: 22357272 Free PMC article.
-
Regulation of Epstein-Barr virus reactivation from latency.Microbiol Immunol. 2014 Jun;58(6):307-17. doi: 10.1111/1348-0421.12155. Microbiol Immunol. 2014. PMID: 24786491 Review.
-
HCF1 and OCT2 Cooperate with EBNA1 To Enhance OriP-Dependent Transcription and Episome Maintenance of Latent Epstein-Barr Virus.J Virol. 2016 May 12;90(11):5353-5367. doi: 10.1128/JVI.00239-16. Print 2016 Jun 1. J Virol. 2016. PMID: 27009953 Free PMC article.
-
The B-cell specific transcription factor, Oct-2, promotes Epstein-Barr virus latency by inhibiting the viral immediate-early protein, BZLF1.PLoS Pathog. 2012 Feb;8(2):e1002516. doi: 10.1371/journal.ppat.1002516. Epub 2012 Feb 9. PLoS Pathog. 2012. PMID: 22346751 Free PMC article.
-
Switching of EBV cycles between latent and lytic states.Rev Med Virol. 2014 May;24(3):142-53. doi: 10.1002/rmv.1780. Epub 2013 Dec 11. Rev Med Virol. 2014. PMID: 24339346 Review.
Cited by
-
Epigenetics and animal virus infections.Front Genet. 2015 Mar 4;6:48. doi: 10.3389/fgene.2015.00048. eCollection 2015. Front Genet. 2015. PMID: 25788901 Free PMC article. No abstract available.
-
Targeting the crosstalk of epigenetic modifications and immune evasion in nasopharyngeal cancer.Cell Biol Toxicol. 2023 Dec;39(6):2501-2526. doi: 10.1007/s10565-023-09830-9. Epub 2023 Sep 27. Cell Biol Toxicol. 2023. PMID: 37755585 Review.
-
The Epstein-Barr Virus BRRF1 Gene Is Dispensable for Viral Replication in HEK293 cells and Transformation.Sci Rep. 2017 Jul 20;7(1):6044. doi: 10.1038/s41598-017-06413-7. Sci Rep. 2017. PMID: 28729695 Free PMC article.
-
PARP1 Inhibition Halts EBV+ Lymphoma Progression by Disrupting the EBNA2/MYC Axis.J Med Virol. 2025 Jul;97(7):e70485. doi: 10.1002/jmv.70485. J Med Virol. 2025. PMID: 40622706 Free PMC article.
-
Dual Role of YY1 in HPV Life Cycle and Cervical Cancer Development.Int J Mol Sci. 2022 Mar 22;23(7):3453. doi: 10.3390/ijms23073453. Int J Mol Sci. 2022. PMID: 35408813 Free PMC article. Review.
References
-
- Amon W., Farrell P. J. (2005). Reactivation of Epstein–Barr virus from latency. Rev. Med. Virol. 15 149–156 - PubMed
-
- Bhende P. M., Seaman W. T., Delecluse H. J., Kenney S. C. (2004). The EBV lytic switch protein, Z, preferentially binds to and activates the methylated viral genome. Nat. Genet. 36 1099–1104 - PubMed
-
- Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., et al. (2002). Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science 298 1039–1043 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

