Differential cytotoxicity responses by dog and rat hepatocytes to phospholipogenic treatments
- PMID: 23577025
- PMCID: PMC3610344
- DOI: 10.1155/2013/956404
Differential cytotoxicity responses by dog and rat hepatocytes to phospholipogenic treatments
Abstract
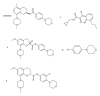
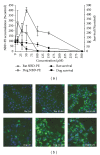
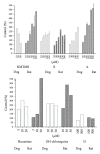
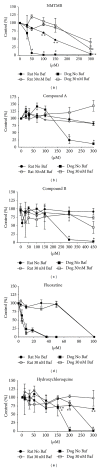
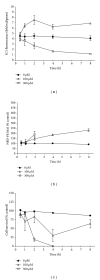
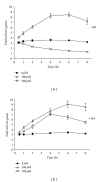
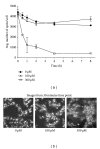
Dog and rat hepatocytes were treated with phospholipogenics to identify the more sensitive species and to determine whether lysosomal or mitochondrial changes were the primary cause of cytotoxicity. Endpoints included cell death, lysosome membrane integrity, mitochondrial membrane polarization, and fluorescent phospholipid (NBD-PE). Dog cells exhibited lower survival IC50 values than did rat cells with all phospholipogenic treatments and exhibited a lower capacity to accumulate NBD-PE in 4 of 5 phospholipogenic test conditions. The lysosomal modulator Bafilomycin A1 (Baf) rescued dog cells from cytotoxicity caused by 3 phospholipogenic 5HT1b antagonists and hydroxychloroquine, but not fluoxetine, and rescued rat cells from hydroxychloroquine and NMTMB, a 5HT1b antagonist. Following NMTMB treatment, rat mitochondrial membrane hyperpolarization was observed at modestly cytotoxic concentrations and depolarization at the highest concentration. At the highest test concentration, lysosomal loss of acridine orange occurred by 30 min, mitochondrial polarity changes by 1 hr, and NBD-PE accumulation by 2 hr, respectively. Baf shifted mitochondrial polarity from a depolarized state to a hyperpolarized state. These data demonstrate that (a) dog hepatocytes were generally less capable of mounting an adaptive, protective phospholipidotic response than rat hepatocytes, (b) effects on mitochondria and survival were preventable by lysosomal protection, and (c) destabilizing changes in both organelles are involved causally in cytotoxicity.
Figures
Similar articles
-
Phospholipidosis assay in HepG2 cells and rat or rhesus hepatocytes using phospholipid probe NBD-PE.Assay Drug Dev Technol. 2008 Jun;6(3):407-19. doi: 10.1089/adt.2007.119. Assay Drug Dev Technol. 2008. PMID: 18537465
-
Phospholipogenic pharmaceuticals are associated with a higher incidence of histological findings than nonphospholipogenic pharmaceuticals in preclinical toxicology studies.J Toxicol. 2012;2012:308594. doi: 10.1155/2012/308594. Epub 2012 Jun 14. J Toxicol. 2012. PMID: 22745636 Free PMC article.
-
A pH probe inhibits senescence in mesenchymal stem cells.Stem Cell Res Ther. 2018 Dec 7;9(1):343. doi: 10.1186/s13287-018-1081-0. Stem Cell Res Ther. 2018. PMID: 30526663 Free PMC article.
-
Mitochondrial dysfunction and biotransformation of β-carboline alkaloids, harmine and harmaline, on isolated rat hepatocytes.Chem Biol Interact. 2010 Dec 5;188(3):393-403. doi: 10.1016/j.cbi.2010.09.004. Epub 2010 Sep 15. Chem Biol Interact. 2010. PMID: 20833158
-
Underlying mechanisms of pharmacology and toxicity of a novel PPAR agonist revealed using rodent and canine hepatocytes.Toxicol Sci. 2007 Apr;96(2):294-309. doi: 10.1093/toxsci/kfm009. Epub 2007 Jan 25. Toxicol Sci. 2007. PMID: 17255113
References
-
- Reasor MJ, Hastings KL, Ulrich RG. Drug-induced phospholipidosis: issues and future directions. Expert Opinion on Drug Safety. 2006;5(4):567–583. - PubMed
-
- Daniel WA, Wójcikowski J. The role of lysosomes in the cellular distribution of thioridazine and potential drug interactions. Toxicology and Applied Pharmacology. 1999;158(2):115–124. - PubMed
-
- Shacka JJ, Klocke BJ, Shibata M, et al. Bafilomycin A1 inhibits chloroquine-induced death of cerebellar granule neurons. Molecular Pharmacology. 2006;69(4):1125–1136. - PubMed
-
- Kornhuber J, Henkel AW, Groemer TW, et al. Lipophilic cationic drugs increase the permeability of lysosomal membranes in a cell culture system. Journal of Cellular Physiology. 2010;224(1):152–164. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

