Quality of clinical practice guidelines for glycemic control in type 2 diabetes mellitus
- PMID: 23577058
- PMCID: PMC3618153
- DOI: 10.1371/journal.pone.0058625
Quality of clinical practice guidelines for glycemic control in type 2 diabetes mellitus
Abstract
Background: Several studies have reported that clinical practice guidelines (CPGs) in a variety of clinical areas are of modest or variable quality. The objective of this study was to evaluate the quality of an international cohort of CPGs that provide recommendations on pharmaceutical management of glycemic control in patients with type 2 diabetes mellitus (DM2).
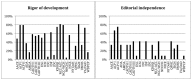
Methods and findings: We searched the National Guideline Clearinghouse (NGC) on February 15th and June 4th, 2012 for CPGs meeting inclusion criteria. Two independent assessors rated the quality of each CPG using the Appraisal of Guidelines for Research & Evaluation II (AGREE II) instrument. Twenty-four guidelines were evaluated, and most had high scores for clarity and presentation. However, scope and purpose, stakeholder involvement, rigor of development, and applicability domains varied considerably. The majority of guidelines scored low on editorial independence, and only seven CPGs were based on an underlying systematic review of the evidence.
Conclusions: The overall quality of CPGs for glycemic control in DM2 is moderate, but there is substantial variability among quality domains within and across guidelines. Guideline users need to be aware of this variability and carefully appraise and select the guidelines that they apply to patient care.
Conflict of interest statement
Figures
Similar articles
-
Quality appraisal of clinical practice guidelines for diabetes mellitus published in China between 2007 and 2017 using the AGREE II instrument.BMJ Open. 2019 Sep 4;9(9):e022392. doi: 10.1136/bmjopen-2018-022392. BMJ Open. 2019. PMID: 31488461 Free PMC article.
-
Guidelines in Low and Middle Income Countries Paper 3: Appraisal of Philippine Clinical Practice Guidelines using Appraisal of Guidelines for Research and Evaluation II: improvement needed for rigor, applicability, and editorial independence.J Clin Epidemiol. 2020 Nov;127:184-190. doi: 10.1016/j.jclinepi.2020.06.036. Epub 2020 Jul 1. J Clin Epidemiol. 2020. PMID: 32621853
-
Systematic Review and Critical Appraisal of Urticaria Clinical Practice Guidelines: A Global Guidelines in Dermatology Mapping Project (GUIDEMAP).J Allergy Clin Immunol Pract. 2023 Oct;11(10):3213-3220.e11. doi: 10.1016/j.jaip.2023.07.002. Epub 2023 Jul 13. J Allergy Clin Immunol Pract. 2023. PMID: 37451615
-
Quantity and quality of complementary and alternative medicine recommendations in clinical practice guidelines for type 2 diabetes mellitus: A systematic review.Nutr Metab Cardiovasc Dis. 2021 Oct 28;31(11):3004-3015. doi: 10.1016/j.numecd.2021.07.029. Epub 2021 Aug 4. Nutr Metab Cardiovasc Dis. 2021. PMID: 34627698
-
The quality of clinical practice guidelines for management of pediatric type 2 diabetes mellitus: a systematic review using the AGREE II instrument.Syst Rev. 2018 Nov 15;7(1):193. doi: 10.1186/s13643-018-0843-1. Syst Rev. 2018. PMID: 30442196 Free PMC article.
Cited by
-
Quality appraisal of gestational diabetes mellitus guidelines with AGREE II: a systematic review.BMC Pregnancy Childbirth. 2019 Dec 5;19(1):478. doi: 10.1186/s12884-019-2597-8. BMC Pregnancy Childbirth. 2019. PMID: 31805878 Free PMC article.
-
A technical appraisal of guidelines for the management of skin rash in patients on chemotherapy and targeted therapy.BMC Health Serv Res. 2019 Oct 16;19(1):704. doi: 10.1186/s12913-019-4539-6. BMC Health Serv Res. 2019. PMID: 31619221 Free PMC article.
-
Guidelines for therapeutic drug monitoring of vancomycin: a systematic review.PLoS One. 2014 Jun 16;9(6):e99044. doi: 10.1371/journal.pone.0099044. eCollection 2014. PLoS One. 2014. PMID: 24932495 Free PMC article.
-
Diagnosis and treatment for hyperuricemia and gout: a systematic review of clinical practice guidelines and consensus statements.BMJ Open. 2019 Aug 24;9(8):e026677. doi: 10.1136/bmjopen-2018-026677. BMJ Open. 2019. PMID: 31446403 Free PMC article.
-
A review of the quality of current diabetes clinical practice guidelines.Rev Panam Salud Publica. 2017 Jun 19;41:e90. doi: 10.26633/RPSP.2017.90. eCollection 2017. Rev Panam Salud Publica. 2017. PMID: 31384248 Free PMC article.
References
-
- Institute of Medicine (2011) Clinical Practice Guidelines We Can Trust; Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E, editors. Washington, D.C.: The National Academies Press. - PubMed
-
- Institute of Medicine (2008) Knowing what works in health care: A roadmap for the nation. Washington, DC: The National Academies Press.
-
- World Health Organization (2012) Diabetes Fact Sheet. Geneva, Switzerland: World Health Organization.
-
- Burda BU, Norris SL, Holmer HK, Ogden LA, Smith BE (2011) Quality varies across clinical practice guidelines for mammography screening in women aged 40–49 years as assessed by AGREE and AMSTAR instruments. J Clinical Epidemiol 64: 968–976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

