Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor
- PMID: 23577075
- PMCID: PMC3618444
- DOI: 10.1371/journal.pone.0059778
Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor
Abstract
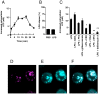
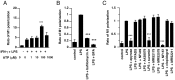
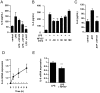
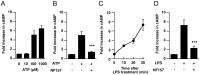
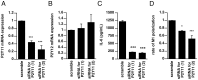
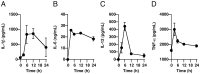
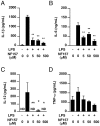
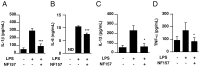
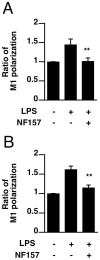
It is important to understand the mechanisms that regulate macrophage activation to establish novel therapies for inflammatory diseases, such as sepsis; a systemic inflammatory response syndrome generally caused by bacterial lipopolysaccharide (LPS). In this study, we investigated the involvement of extracellular ATP-mediated autocrine signaling in LPS-induced macrophage activation. We show here that ATP release via exocytosis, followed by activation of P2Y11 receptor, is a major pathway of the macrophage activation, leading to release of cytokines. Treatment of human monocyte THP-1 cells with LPS induced rapid ATP release from cells, and this release was blocked by knockdown of SLC17A9 (vesicular nucleotide transporter, VNUT), which is responsible for exocytosis of ATP. ATP-enriched vesicles were found in cytosol of THP-1 cells. These data suggest the involvement of vesicular exocytosis in the release of ATP. Knockdown of SLC17A9, the P2Y11 antagonist NF157 or knockdown of P2Y11 receptor significantly suppressed both M1-type polarization and IL-6 production in THP-1 cells, indicating an important role of activation of P2Y11 receptor by released ATP in macrophage activation. Next, the effect of NF157 on LPS-induced immune activation was examined in vivo. Administration of LPS to mice caused increase of serum IL-1ß, IL-6, IL-12 and TNF-alpha levels at 3-24 h after the administration. Pre-treatment of LPS-treated mice with NF157 suppressed both elevation of proinflammatory cytokines in serum and M1 polarization of peritoneal/spleen macrophages. Moreover, post-treatment with NF157 at 30 min after administration of LPS also suppressed the elevation of serum cytokines levels. We conclude that vesicular exocytosis of ATP and autocrine, positive feedback through P2Y11 receptors is required for the effective activation of macrophages. Consequently, P2Y11 receptor antagonists may be drug candidates for treatment of inflammatory diseases such as sepsis.
Conflict of interest statement
Figures

Similar articles
-
Autocrine regulation of TGF-β1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells.J Cell Sci. 2012 Nov 1;125(Pt 21):5051-60. doi: 10.1242/jcs.104976. Epub 2012 Sep 3. J Cell Sci. 2012. PMID: 22946048
-
Lipopolysaccharide suppresses T cells by generating extracellular ATP that impairs their mitochondrial function via P2Y11 receptors.J Biol Chem. 2019 Apr 19;294(16):6283-6293. doi: 10.1074/jbc.RA118.007188. Epub 2019 Feb 20. J Biol Chem. 2019. PMID: 30787105 Free PMC article.
-
Involvement of P2Y₁₁ receptor in IFN-γ-induced IL-6 production in human keratinocytes.Eur J Pharmacol. 2013 Mar 5;703(1-3):67-73. doi: 10.1016/j.ejphar.2013.02.020. Epub 2013 Feb 24. Eur J Pharmacol. 2013. PMID: 23461851
-
Signal transduction and white cell maturation via extracellular ATP and the P2Y11 receptor.Immunol Cell Biol. 2000 Aug;78(4):369-74. doi: 10.1046/j.1440-1711.2000.00918.x. Immunol Cell Biol. 2000. PMID: 10947861 Review.
-
P2Y11/IL-1 receptor crosstalk controls macrophage inflammation: a novel target for anti-inflammatory strategies?Purinergic Signal. 2023 Sep;19(3):501-511. doi: 10.1007/s11302-023-09932-3. Epub 2023 Apr 5. Purinergic Signal. 2023. PMID: 37016172 Free PMC article. Review.
Cited by
-
Drug-triggered and cross-linked self-assembling nanofibrous hydrogels.J Am Chem Soc. 2015 Apr 15;137(14):4823-30. doi: 10.1021/jacs.5b01549. Epub 2015 Apr 1. J Am Chem Soc. 2015. PMID: 25831137 Free PMC article.
-
Rhus Coriaria L. Extract: Antioxidant Effect and Modulation of Bioenergetic Capacity in Fibroblasts from Parkinson's Disease Patients and THP-1 Macrophages.Int J Mol Sci. 2022 Oct 23;23(21):12774. doi: 10.3390/ijms232112774. Int J Mol Sci. 2022. PMID: 36361562 Free PMC article.
-
Purinergic signalling and immune cells.Purinergic Signal. 2014 Dec;10(4):529-64. doi: 10.1007/s11302-014-9427-2. Epub 2014 Oct 29. Purinergic Signal. 2014. PMID: 25352330 Free PMC article. Review.
-
Release mechanisms of major DAMPs.Apoptosis. 2021 Apr;26(3-4):152-162. doi: 10.1007/s10495-021-01663-3. Epub 2021 Mar 13. Apoptosis. 2021. PMID: 33713214 Free PMC article. Review.
-
The Human G Protein-Coupled ATP Receptor P2Y11 Is Associated With IL-10 Driven Macrophage Differentiation.Front Immunol. 2019 Aug 9;10:1870. doi: 10.3389/fimmu.2019.01870. eCollection 2019. Front Immunol. 2019. PMID: 31447857 Free PMC article.
References
-
- Cohen J (2002) The immunopathogenesis of sepsis. Nature 420: 885–891. - PubMed
-
- Kilbourn RG, Griffith OW, Gross SS (1993) Pathogenetic mechanisms of septic shock. N Engl J Med 329: 1427–1428. - PubMed
-
- Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150. - PubMed
-
- Glauser MP (1996) The inflammatory cytokines. New developments in the pathophysiology and treatment of septic shock. Drugs 52 Suppl 29–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

