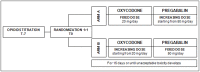
Randomised phase II trial (NCT00637975) evaluating activity and toxicity of two different escalating strategies for pregabalin and oxycodone combination therapy for neuropathic pain in cancer patients
- PMID: 23577077
- PMCID: PMC3618180
- DOI: 10.1371/journal.pone.0059981
Randomised phase II trial (NCT00637975) evaluating activity and toxicity of two different escalating strategies for pregabalin and oxycodone combination therapy for neuropathic pain in cancer patients
Abstract
Purpose: Neuropathic pain is commonly associated with cancer. Current treatments include combination opioid and adjuvant therapies, but no guidelines are available for dose escalation strategies. This phase II study compared the efficacy and tolerability of two dose escalation strategies for oxycodone and pregabalin combination therapy.
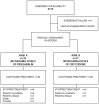
Methods: Patients (N = 75) with oncological neuropathic pain, previously untreated with pregabalin, were recruited in 5 Italian institutions between 2007 and 2010. Patients were randomised to two different dose escalation strategies (arm A; N = 38) oxycodone at a fixed dose with increasing pregabalin doses; (arm B; N = 37) pregabalin at a fixed dose with increasing oxycodone doses. Patients were evaluated from daily diaries and follow-ups at 3, 7, 10, and 14 days after beginning treatment with a numerical rating scale (NRS), neuropathic pain scale (SDN), and well-being scale (ESAS). The primary endpoint was a ≥1/3 reduction in pain (NRS); secondary endpoints included the time to analgesia and adverse effects. The study had a 90% probability of detecting the best strategy for a true difference of at least 15%.
Results: More patients in arm A (76%) than arm B (64%) achieved ≥1/3 overall pain reduction even after controlling for baseline factors (gender, baseline pain). Group A reported fewer side effects than group B; constipation 52.8% vs. 66.7%; nausea: 27.8% vs. 44.4%; drowsiness: 44.4% vs. 55.6%; confusion: 16.7% vs. 27.8%; itching: 8.3% vs. 19.4%.
Conclusions: Both strategies effectively controlled neuropathic pain, but according to the adopted selection design arm A is preferable to arm B for pain control.
Trial registration: ClinicalTrials.gov NCT00637975.
Conflict of interest statement
Figures
References
-
- Irving GA (2005) Contemporary assessment and management of neuropathic pain. Neurology 64(12 Suppl 3)S21–7. - PubMed
-
- Eisenberg E, McNicol ED, Carr DB (2005) Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trials. JAMA 293(24): 3043–52. - PubMed
-
- Bruera E, Belzile M, Pituskin E, Fainsinger R, Darke A, et al. (1998) Randomized, double-blind, cross-over trial comparing safety and efficacy of oral controlled-release oxycodone with controlled-release morphine in patients with cancer pain. J Clin Oncol 16(10): 3222–9. - PubMed
-
- Portenoy R (1998) Ajuvant analgesics in pain management. In: Doyle D HG (MacDonald N editor) Oxford Textbook of Palliative Medicine, ed. Oxford, Oxford University Press p. 361–390.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical