Paralogous ribosomal protein l32-1 and l32-2 in fission yeast may function distinctively in cellular proliferation and quiescence by changing the ratio of rpl32 paralogs
- PMID: 23577148
- PMCID: PMC3618328
- DOI: 10.1371/journal.pone.0060689
Paralogous ribosomal protein l32-1 and l32-2 in fission yeast may function distinctively in cellular proliferation and quiescence by changing the ratio of rpl32 paralogs
Abstract
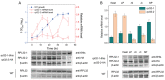
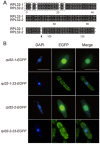
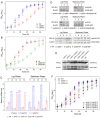
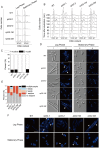
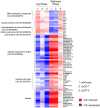
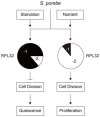
Fission yeast cells express Rpl32-2 highly while Rpl32-1 lowly in log phase; in contrast, expression of Rpl32-1 raises and reaches a peak level while Rpl32-2 is downregulated to a low basic level when cells enter into stationary phase. Overexpression of Rpl32-1 inhibits cell growth while overexpression of Rpl32-2 does not. Deleting rpl32-2 impairs cell growth more severely than deleting rpl32-1 does. Cell growth impaired by deleting either paralog can be rescued completely by reintroducing rpl32-2, but only partly by rpl32-1. Overexpression of Rpl32-1 inhibits cell division, yielding 4c DNA and multiple septa, while overexpressed Rpl32-2 promotes it. Transcriptomics analysis proved that Rpl32 paralogs regulate expression of a subset of genes related with cell division and stress response in a distinctive way. This functional difference of the two paralogs is due to their difference of 95(th) amino acid residue. The significance of a competitive inhibition between Rpl32 paralogs on their expression is discussed.
Conflict of interest statement
Figures
Similar articles
-
Reduction of ribosome level triggers flocculation of fission yeast cells.Eukaryot Cell. 2013 Mar;12(3):450-9. doi: 10.1128/EC.00321-12. Epub 2013 Jan 25. Eukaryot Cell. 2013. PMID: 23355005 Free PMC article.
-
Characterization of the non-sexual flocculation of fission yeast cells that results from the deletion of ribosomal protein L32.Yeast. 2015 May;32(5):439-49. doi: 10.1002/yea.3070. Epub 2015 Mar 11. Yeast. 2015. PMID: 25704380
-
The ribosomal protein L32-2 (RPL32-2) of S. pombe exhibits a novel extraribosomal function by acting as a potential transcriptional regulator.FEBS Lett. 2006 Mar 20;580(7):1827-32. doi: 10.1016/j.febslet.2006.02.040. Epub 2006 Feb 24. FEBS Lett. 2006. PMID: 16516201
-
Global control of cell growth in fission yeast and its coordination with the cell cycle.Curr Opin Cell Biol. 2012 Dec;24(6):833-7. doi: 10.1016/j.ceb.2012.10.015. Epub 2012 Nov 22. Curr Opin Cell Biol. 2012. PMID: 23182517 Review.
-
Isolation and characterization of fission yeast genes involved in transcription regulation of cell cycle events (a short communication).Acta Microbiol Immunol Hung. 2002;49(2-3):285-7. doi: 10.1556/AMicr.49.2002.2-3.16. Acta Microbiol Immunol Hung. 2002. PMID: 12109160 Review. No abstract available.
Cited by
-
Ribosomal protein L32 enhances hepatocellular carcinoma progression.Cancer Med. 2023 May;12(9):10791-10803. doi: 10.1002/cam4.5811. Epub 2023 Apr 5. Cancer Med. 2023. PMID: 37017565 Free PMC article.
References
-
- Ohno S (1970) Evolution by gene duplication. Berlin: Springer Verlag.
-
- Lynch M, Conery JS (2000) The evolutionary fate and consequences of duplicate genes. Science 290: 1151–1155. - PubMed
-
- Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R, et al. (2002) The genome sequence of Schizosaccharomyces pombe . Nature 415: 871–880. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

