Birbeck granule-like "organized smooth endoplasmic reticulum" resulting from the expression of a cytoplasmic YFP-tagged langerin
- PMID: 23577166
- PMCID: PMC3618057
- DOI: 10.1371/journal.pone.0060813
Birbeck granule-like "organized smooth endoplasmic reticulum" resulting from the expression of a cytoplasmic YFP-tagged langerin
Abstract
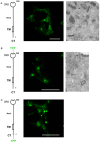
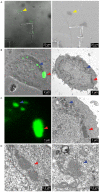
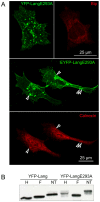
Langerin is required for the biogenesis of Birbeck granules (BGs), the characteristic organelles of Langerhans cells. We previously used a Langerin-YFP fusion protein having a C-terminal luminal YFP tag to dynamically decipher the molecular and cellular processes which accompany the traffic of Langerin. In order to elucidate the interactions of Langerin with its trafficking effectors and their structural impact on the biogenesis of BGs, we generated a YFP-Langerin chimera with an N-terminal, cytosolic YFP tag. This latter fusion protein induced the formation of YFP-positive large puncta. Live cell imaging coupled to a fluorescence recovery after photobleaching approach showed that this coalescence of proteins in newly formed compartments was static. In contrast, the YFP-positive structures present in the pericentriolar region of cells expressing Langerin-YFP chimera, displayed fluorescent recovery characteristics compatible with active membrane exchanges. Using correlative light-electron microscopy we showed that the coalescent structures represented highly organized stacks of membranes with a pentalaminar architecture typical of BGs. Continuities between these organelles and the rough endoplasmic reticulum allowed us to identify the stacks of membranes as a form of "Organized Smooth Endoplasmic Reticulum" (OSER), with distinct molecular and physiological properties. The involvement of homotypic interactions between cytoplasmic YFP molecules was demonstrated using an A206K variant of YFP, which restored most of the Langerin traffic and BG characteristics observed in Langerhans cells. Mutation of the carbohydrate recognition domain also blocked the formation of OSER. Hence, a "double-lock" mechanism governs the behavior of YFP-Langerin, where asymmetric homodimerization of the YFP tag and homotypic interactions between the lectin domains of Langerin molecules participate in its retention and the subsequent formation of BG-like OSER. These observations confirm that BG-like structures appear wherever Langerin accumulates and confirm that membrane trafficking effectors dictate their physiology and, illustrate the importance of molecular interactions in the architecture of intracellular membranes.
Conflict of interest statement
Figures



References
-
- Shimomura O, Johnson FH, Saiga Y (1962) Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol 59: 223–239. - PubMed
-
- Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ (1992) Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111: 229–233. - PubMed
-
- Zacharias DA, Violin JD, Newton AC, Tsien RY (2002) Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296: 913–916. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

