Overexpression of full-length centrobin rescues limb malformation but not male fertility of the hypodactylous (hd) rats
- PMID: 23577170
- PMCID: PMC3620055
- DOI: 10.1371/journal.pone.0060859
Overexpression of full-length centrobin rescues limb malformation but not male fertility of the hypodactylous (hd) rats
Abstract
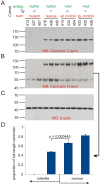
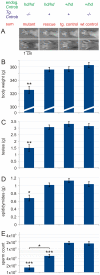
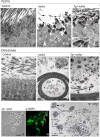
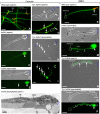
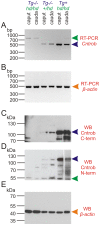
Rat hypodactyly (hd) mutation is characterized by abnormal spermatogenesis and sperm decapitation, limb malformation (missing digits II and III) and growth retardation. We have previously reported centrobin (centrosome BRCA2-interacting protein) truncation at the C-terminus in the hd mutant. Here, we report data from a transgenic rescue experiment carried out to determine a role of centrobin in pathogenesis of hd. The transgenic construct, consisting of full-length-coding cDNA linked to a ubiquitous strong promoter/enhancer combination, was inserted to chromosome 16 into a LINE repeat. No known gene is present in the vicinity of the insertion site. Transgenic centrobin was expressed in all tissues tested, including testis. Transgenic animals show normal body weight and limb morphology as well as average weight of testis and epididymis. Yet, abnormal spermatogenesis and sperm decapitation persisted in the transgenic animals. Western blotting showed the coexistence of full-length and truncated or partially degraded centrobin in sperm of the rescued transgenic animals. Immunocytochemistry showed a buildup of centrobin and ODF2 (outer dense fiber 2) at the sperm decapitation site in the hd mutant and rescued transgenic rats. Additional findings included bulge-like formations and thread-like focal dissociations along the sperm flagellum and the organization of multiple whorls of truncated sperm flagella in the epididymal lumen. We conclude that centrobin is essential for normal patterning of the limb autopod. Centrobin may be required for stabilizing the attachment of the sperm head to flagellum and for maintaining the structural integrity of the sperm flagellum. We postulate that the presence of truncated centrobin, coexisting with full-length centrobin, together with incorrect timing of transgenic centrobin expression may hamper the rescue of fertility in hd male rats.
Conflict of interest statement
Figures

Similar articles
-
Rat hd mutation reveals an essential role of centrobin in spermatid head shaping and assembly of the head-tail coupling apparatus.Biol Reprod. 2009 Dec;81(6):1196-205. doi: 10.1095/biolreprod.109.078980. Epub 2009 Aug 26. Biol Reprod. 2009. PMID: 19710508 Free PMC article.
-
CCDC38 is required for sperm flagellum biogenesis and male fertility in mice.Development. 2022 Jun 1;149(11):dev200516. doi: 10.1242/dev.200516. Epub 2022 Jun 13. Development. 2022. PMID: 35587122
-
Severe limb defects in Hypodactyly mice result from the expression of a novel, mutant HOXA13 protein.Dev Biol. 2000 Jan 15;217(2):290-300. doi: 10.1006/dbio.1999.9550. Dev Biol. 2000. PMID: 10625554
-
Expression of transgenic PPP1CC2 in the testis of Ppp1cc-null mice rescues spermatid viability and spermiation but does not restore normal sperm tail ultrastructure, sperm motility, or fertility.Biol Reprod. 2009 Aug;81(2):343-52. doi: 10.1095/biolreprod.109.076398. Epub 2009 May 6. Biol Reprod. 2009. PMID: 19420386 Free PMC article.
-
Tssk4 is essential for maintaining the structural integrity of sperm flagellum.Mol Hum Reprod. 2015 Feb;21(2):136-45. doi: 10.1093/molehr/gau097. Epub 2014 Oct 31. Mol Hum Reprod. 2015. PMID: 25361759
Cited by
-
TRIM37 prevents formation of condensate-organized ectopic spindle poles to ensure mitotic fidelity.J Cell Biol. 2021 Jul 5;220(7):e202010180. doi: 10.1083/jcb.202010180. Epub 2021 May 13. J Cell Biol. 2021. PMID: 33983387 Free PMC article.
-
Centrobin is essential for C-tubule assembly and flagellum development in Drosophila melanogaster spermatogenesis.J Cell Biol. 2018 Jul 2;217(7):2365-2372. doi: 10.1083/jcb.201801032. Epub 2018 Apr 30. J Cell Biol. 2018. PMID: 29712734 Free PMC article.
-
The sperm centrioles.Mol Cell Endocrinol. 2020 Dec 1;518:110987. doi: 10.1016/j.mce.2020.110987. Epub 2020 Aug 15. Mol Cell Endocrinol. 2020. PMID: 32810575 Free PMC article. Review.
-
The Transformation of the Centrosome into the Basal Body: Similarities and Dissimilarities between Somatic and Male Germ Cells and Their Relevance for Male Fertility.Cells. 2021 Aug 31;10(9):2266. doi: 10.3390/cells10092266. Cells. 2021. PMID: 34571916 Free PMC article. Review.
-
Centrobin controls primary ciliogenesis in vertebrates.J Cell Biol. 2018 Apr 2;217(4):1205-1215. doi: 10.1083/jcb.201706095. Epub 2018 Feb 13. J Cell Biol. 2018. PMID: 29440264 Free PMC article.
References
-
- Boivin J, Bunting L, Collins JA, Nygren KG (2007) International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 22: 1506–1512. - PubMed
-
- Liška F (2003) Selected genetic aspects of male infertility–what animal models tell us. Folia Biol (Praha) 49: 129–141. - PubMed
-
- Manandhar G, Simerly C, Schatten G (2000) Centrosome reduction during mammalian spermiogenesis. Curr Top Dev Biol 49: 343–363. - PubMed
-
- Woolley DM, Fawcett DW (1973) The degeneration and disappearance of the centrioles during the development of the rat spermatozoon. Anat Rec 177: 289–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

