Oxidative stress and mitogen-activated protein kinase pathways involved in cadmium-induced BRL 3A cell apoptosis
- PMID: 23577223
- PMCID: PMC3618937
- DOI: 10.1155/2013/516051
Oxidative stress and mitogen-activated protein kinase pathways involved in cadmium-induced BRL 3A cell apoptosis
Abstract
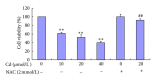
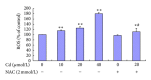
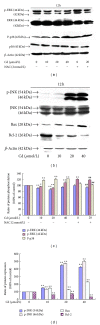
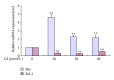
In this study, BRL 3A cells were treated with different Cd concentrations (0, 10, 20, and 40 μmol/L) for 12 h and preincubated with or without N-acetyl-L-cysteine (NAC) (2 mmol/L) for 30 min, and cells were treated with Cd (0 and 20 μmol/L), pretreated with p38 inhibitor (SB203580), JNK (c-Jun NH2-terminal kinases) inhibitor (SP600125), and extracellular signal-regulated kinase (ERK) inhibitor (U0126) for 30 min, and then treated with 20 μmol/L Cd for 12 h. Cd decreased cell viability, SOD, and GSH-Px activity in a concentration-dependent manner. Increased MDA level, ROS generation, nuclear condensation, shrinkage, and fragmentation in cell morphology were inhibited by NAC. Cd-induced apoptosis was attenuated by pretreatment with SB203580, SP600125, and U0126. The results of western blot showed that NAC preincubation affected Cd-activated MAPK pathways, p38 and ERK phosphorylation. Cd treatment elevated the mRNA levels of Bax and decreased the mRNA levels of Bcl-2, respectively. The same effect was found in their protein expression levels. These results suggest that oxidative stress and MAPK pathways participate in Cd-induced apoptosis and that the balance between pro- and antiapoptotic genes (Bax and Bcl-2) is important in Cd-induced apoptosis.
Figures



References
-
- Méndez-Armenta M, Ríos C. Cadmium neurotoxicity. Environmental Toxicology and Pharmacology. 2007;23(3):350–358. - PubMed
-
- Bertin G, Averbeck D. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review) Biochimie. 2006;88(11):1549–1559. - PubMed
-
- Thompson J, Doi T, Power E, Balasubramanian I, Puri P, Bannigan J. Evidence against a direct role for oxidative stress in cadmium-induced axial malformation in the chick embryo. Toxicology and Applied Pharmacology. 2010;243(3):390–398. - PubMed
-
- Brzóska MM, Rogalska J, Kupraszewicz E. The involvement of oxidative stress in the mechanisms of damaging cadmium action in bone tissue: a study in a rat model of moderate and relatively high human exposure. Toxicology and Applied Pharmacology. 2011;250(3):327–335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

