Molecular modeling and ligand docking for solute carrier (SLC) transporters
- PMID: 23578028
- PMCID: PMC4056341
- DOI: 10.2174/1568026611313070007
Molecular modeling and ligand docking for solute carrier (SLC) transporters
Abstract
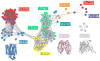
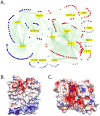
Solute Carrier (SLC) transporters are membrane proteins that transport solutes, such as ions, metabolites, peptides, and drugs, across biological membranes, using diverse energy coupling mechanisms. In human, there are 386 SLC transporters, many of which contribute to the absorption, distribution, metabolism, and excretion of drugs and/or can be targeted directly by therapeutics. Recent atomic structures of SLC transporters determined by X-ray crystallography and NMR spectroscopy have significantly expanded the applicability of structure-based prediction of SLC transporter ligands, by enabling both comparative modeling of additional SLC transporters and virtual screening of small molecules libraries against experimental structures as well as comparative models. In this review, we begin by describing computational tools, including sequence analysis, comparative modeling, and virtual screening, that are used to predict the structures and functions of membrane proteins such as SLC transporters. We then illustrate the applications of these tools to predicting ligand specificities of select SLC transporters, followed by experimental validation using uptake kinetic measurements and other assays. We conclude by discussing future directions in the discovery of the SLC transporter ligands.
Figures



References
-
- Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004;447(5):465–468. - PubMed
-
- Povey S, Lovering R, Bruford E, Wright M, Lush M, Wain H. The HUGO Gene Nomenclature Committee (HGNC) Hum Genet. 2001;109(6):678–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

