Multiple therapeutic peptide vaccines consisting of combined novel cancer testis antigens and anti-angiogenic peptides for patients with non-small cell lung cancer
- PMID: 23578144
- PMCID: PMC3639131
- DOI: 10.1186/1479-5876-11-97
Multiple therapeutic peptide vaccines consisting of combined novel cancer testis antigens and anti-angiogenic peptides for patients with non-small cell lung cancer
Abstract
Background: Vaccine treatment using multiple peptides derived from multiple proteins is considered to be a promising option for cancer immune therapy, but scientific evidence supporting the therapeutic efficacy of multiple peptides is limited.
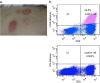
Methods: We conducted phase I trials using a mixture of multiple therapeutic peptide vaccines to evaluate their safety, immunogenicity and clinical response in patients with advanced/recurrent NSCLC. We administered two different combinations of four HLA-A24-restricted peptides. Two were peptides derived from vascular endothelial growth factor receptor 1 (VEGFR1) and 2 (VEGFR2), and the third was a peptide derived from up-regulated lung cancer 10 (URLC10, which is also called lymphocyte antigen 6 complex locus K [LY6K]). The fourth peptide used was derived from TTK protein kinase (TTK) or cell division associated 1 (CDCA1). Vaccines were administered weekly by subcutaneous injection into the axillary region of patients with montanide ISA-51 incomplete Freund's adjuvant, until the disease was judged to have progressed or patients requested to be withdrawn from the trial. Immunological responses were primarily evaluated using an IFN-gamma ELiSPOT assay.
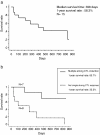
Results: Vaccinations were well tolerated with no severe treatment-associated adverse events except for the reactions that occurred at the injection sites. Peptide-specific T cell responses against at least one peptide were observed in 13 of the 15 patients enrolled. Although no patient exhibited complete or partial responses, seven patients (47%) had stable disease for at least 2 months. The median overall survival time was 398 days, and the 1- and 2-year survival rates were 58.3% and 32.8%, respectively.
Conclusion: Peptide vaccine therapy using a mixture of four novel peptides was found to be safe, and is expected to induce strong specific T cell responses.
Trial registration: These studies were registered with ClinicalTrials.gov NCT00633724 and NCT00874588.
Figures
References
-
- Vansteenkiste J, Zielinski M, Linder A, Dahabre J, Esteban E, Malinowski W, Jassem J, Passlick B, Lehmann F, Brichard VG. Final results of a multi-center, double-blind, randomized, placebo-controlled phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol. 2007;25:s7554.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

