Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits
- PMID: 23578170
- PMCID: PMC3809700
- DOI: 10.1111/imm.12110
Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits
Abstract
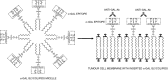
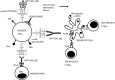
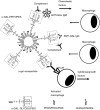
Anti-Gal is the most abundant natural antibody in humans, constituting ~ 1% of immunoglobulins. Anti-Gal is naturally produced also in apes and Old World monkeys. The ligand of anti-Gal is a carbohydrate antigen called the 'α-gal epitope' with the structure Galα1-3Galβ1-4GlcNAc-R. The α-gal epitope is present as a major carbohydrate antigen in non-primate mammals, prosimians and New World monkeys. Anti-Gal can contributes to several immunological pathogeneses. Anti-Gal IgE produced in some individuals causes allergies to meat and to the therapeutic monoclonal antibody cetuximab, all presenting α-gal epitopes. Aberrant expression of the α-gal epitope or of antigens mimicking it in humans may result in autoimmune processes, as in Graves' disease. α-Gal epitopes produced by Trypanosoma cruzi interact with anti-Gal and induce 'autoimmune like' inflammatory reactions in Chagas' disease. Anti-Gal IgM and IgG further mediate rejection of xenografts expressing α-gal epitopes. Because of its abundance, anti-Gal may be exploited for various clinical uses. It increases immunogenicity of microbial vaccines (e.g. influenza vaccine) presenting α-gal epitopes by targeting them for effective uptake by antigen-presenting cells. Tumour lesions are converted into vaccines against autologous tumour-associated antigens by intra-tumoral injection of α-gal glycolipids, which insert into tumour cell membranes. Anti-Gal binding to α-gal epitopes on tumour cells targets them for uptake by antigen-presenting cells. Accelerated wound healing is achieved by application of α-gal nanoparticles, which bind anti-Gal, activate complement, and recruit and activate macrophages that induce tissue regeneration. This therapy may be of further significance in regeneration of internally injured tissues such as ischaemic myocardium and injured nerves.
Keywords: anti-Gal; increased immunogenicity; tumour vaccine; wound healing; α-gal epitopes.
© 2013 John Wiley & Sons Ltd.
Figures
References
-
- Galili U, Anaraki F, Thall A, Hill-Black C, Radic M. One percent of circulating B lymphocytes are capable of producing the natural anti-Gal antibody. Blood. 1993;82:2485–93. - PubMed
-
- Wang L, Radic MZ, Galili U. Human anti-Gal heavy chain genes: preferential use of VH3 and the presence of somatic mutations. J Immunol. 1995;155:1276–85. - PubMed
-
- Kearns-Jonker M, Swensson J, Ghiuzeli C, et al. The human antibody response to porcine xenoantigens is encoded by IGHV3–11 and IGHV3–74 IgVH germline progenitors. J Immunol. 1999;163:4399–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

