Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway
- PMID: 23578591
- PMCID: PMC3672366
- DOI: 10.1016/j.jaut.2013.03.002
Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway
Abstract
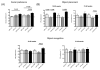
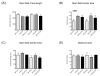
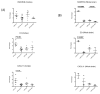
Given the early onset of neuropsychiatric disease and the potential response to immunosuppressive therapy, neuropsychiatric disease is considered a primary disease manifestation in systemic lupus erythematosus (SLE). However, the pathogenesis is not fully understood and optimal treatment has yet to be determined. TWEAK is a TNF family ligand that mediates pleotropic effects through its receptor Fn14, including the stimulation of inflammatory cytokine production by astrocytes, endothelial cells, and other non-hematopeotic cell types, and induction of neuronal death. Furthermore, TWEAK-inducible mediators are implicated in neuropsychiatric lupus. Thus, we hypothesized that the TWEAK/Fn14 pathway may be involved in the pathogenesis of neuropsychiatric SLE. We generated MRL-lpr/lpr (MRL/lpr) mice deficient for Fn14, the sole known signaling receptor for TWEAK. Neuropsychiatric disease was compared in age- and gender-matched MRL/lpr Fn14 wild type (WT) and knockout (KO) mice, using a comprehensive battery of neurobehavioral tests. We found that MRL/lpr Fn14WT mice displayed profound depression-like behavior as seen by increased immobility in a forced swim test and loss of preference for sweetened fluids, which were significantly ameliorated in Fn14KO mice. Similarly, MRL/lpr Fn14WT mice had impaired cognition, and this was significantly improved in Fn14KO mice. To determine the mechanism by which Fn14 deficiency ameliorates neuropsychiatric disease, we assessed the serum levels of autoantibodies and local expression of cytokines in the cortex and hippocampus of lupus mice. No significant differences were found in the serum levels of antibodies to nuclear antigens, or autoantibodies specifically associated with neuropsychiatric disease, between MRL/lpr Fn14WT and KO mice. However, MRL/lpr Fn14KO mice had significantly decreased brain expression of RANTES, C3, and other proinflammatory mediators. Furthermore, MRL/lpr Fn14KO mice displayed improved blood brain barrier integrity. In conclusion, several central manifestations of neuropsychiatric lupus, including depression-like behavior and altered cognition, are normalized in MRL/lpr mice lacking Fn14. Our results are the first to indicate a role for the TWEAK/Fn14 pathway in the pathogenesis of neuropsychiatric lupus, and suggest this ligand-receptor pair as a potential therapeutic target for a common and dangerous disease manifestation.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures






References
-
- The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. - PubMed
-
- Petri M, Naqibuddin M, Carson KA, Wallace DJ, Weisman MH, Holliday SL, et al. Depression and cognitive impairment in newly diagnosed systemic lupus erythematosus. J Rheumatol. 2010;37:2032–38. - PubMed
-
- Sakic B, Denburg JA, Denburg SD, Szechtman H. Blunted sensitivity to sucrose in autoimmune MRL-lpr mice: a curve-shift study. Brain Res Bull. 1996;41:305–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

