MR imaging features of amyloid-related imaging abnormalities
- PMID: 23578674
- PMCID: PMC7965435
- DOI: 10.3174/ajnr.A3500
MR imaging features of amyloid-related imaging abnormalities
Abstract
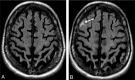
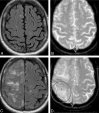
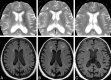
Background and purpose: AD is one of the few leading causes of death without a disease-modifying drug; however, hopeful agents are in various phases of development. MR imaging abnormalities, collectively referred to as amyloid-related imaging abnormalities, have been reported for several agents that target cerebral Aβ burden. ARIA includes ARIA-E, parenchymal or sulcal hyperintensities on FLAIR indicative of parenchymal edema or sulcal effusions, and ARIA-H, hypointense regions on gradient recalled-echo/T2* indicative of hemosiderin deposition. This report describes imaging characteristics of ARIA-E and ARIA-H identified during studies of bapineuzumab, a humanized monoclonal antibody against Aβ.
Materials and methods: Two neuroradiologists with knowledge of imaging changes reflective of ARIA reviewed MR imaging scans from 210 bapineuzumab-treated patients derived from 3 phase 2 studies. Each central reader interpreted the studies independently, and discrepancies were resolved by consensus. The inter-reader κ was 0.76, with 94% agreement between neuroradiologists regarding the presence or absence of ARIA-E in individual patients.
Results: Thirty-six patients were identified with incident ARIA-E (17.1%, 36/210) and 26 with incident ARIA-H (12.4%, 26/210); of those with incident ARIA-H, 24 had incident microhemorrhages and 2 had incident large superficial hemosiderin deposits.
Conclusions: In 49% of cases of ARIA-E, there was the associated appearance of ARIA-H. In treated patients without ARIA-E, the risk for incident blood products was 4%. This association between ARIA-E and ARIA-H may suggest a common pathophysiologic mechanism. Familiarity with ARIA should permit radiologists and clinicians to recognize and communicate ARIA findings more reliably for optimal patient management.
Figures


References
-
- Sperling R, Bronen R, Greenberg S, et al. . Three cases of apparent vasogenic edema (VE) from a phase 2 clinical trial of the gamma secretase inhibitor BMS-708163 in patients with mild-to-moderate AD. Alzheimers Dement 2011;7(suppl):S377
-
- Doody R. Results from independent analyses of the EXPEDITION (solanezumab) study data conducted by the Alzheimer's Disease Cooperative Study (ADCS). In: Proceedings of the Annual Meeting of The American Neurological Association, Boston, Massachusetts. October 7–9, 2012
-
- Ostrowitzki S, Deptula D, Thurfjell L, et al. . Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol 2012;69:198–207 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
