The cataract and glucosuria associated monocarboxylate transporter MCT12 is a new creatine transporter
- PMID: 23578822
- PMCID: PMC3723308
- DOI: 10.1093/hmg/ddt175
The cataract and glucosuria associated monocarboxylate transporter MCT12 is a new creatine transporter
Abstract
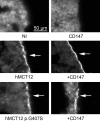
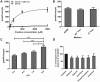
Creatine transport has been assigned to creatine transporter 1 (CRT1), encoded by mental retardation associated SLC6A8. Here, we identified a second creatine transporter (CRT2) known as monocarboxylate transporter 12 (MCT12), encoded by the cataract and glucosuria associated gene SLC16A12. A non-synonymous alteration in MCT12 (p.G407S) found in a patient with age-related cataract (ARC) leads to a significant reduction of creatine transport. Furthermore, Slc16a12 knockout (KO) rats have elevated creatine levels in urine. Transport activity and expression characteristics of the two creatine transporters are distinct. CRT2 (MCT12)-mediated uptake of creatine was not sensitive to sodium and chloride ions or creatine biosynthesis precursors, breakdown product creatinine or creatine phosphate. Increasing pH correlated with increased creatine uptake. Michaelis-Menten kinetics yielded a Vmax of 838.8 pmol/h/oocyte and a Km of 567.4 µm. Relative expression in various human tissues supports the distinct mutation-associated phenotypes of the two transporters. SLC6A8 was predominantly found in brain, heart and muscle, while SLC16A12 was more abundant in kidney and retina. In the lens, the two transcripts were found at comparable levels. We discuss the distinct, but possibly synergistic functions of the two creatine transporters. Our findings infer potential preventive power of creatine supplementation against the most prominent age-related vision impaired condition.
Figures


References
-
- Walker J.B. Creatine: biosynthesis, regulation, and function. Adv. Enzymol. Relat. Areas Mol. Biol. 1979;50:177–242. - PubMed
-
- Wallimann T., Wyss M., Brdiczka D., Nicolay K., Eppenberger H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992;281(Pt 1):21–40. - PMC - PubMed
-
- Wallimann T., Tokarska-Schlattner M., Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40:1271–1296. doi:10.1007/s00726-011-0877-3. - DOI - PMC - PubMed
-
- Fitch C.D., Shields R.P., Payne W.F., Dacus J.M. Creatine metabolism in skeletal muscle. 3. Specificity of the creatine entry process. J. Biol. Chem. 1968;243:2024–2027. - PubMed
-
- Dai W., Vinnakota S., Qian X., Kunze D.L., Sarkar H.K. Molecular characterization of the human CRT-1 creatine transporter expressed in Xenopus oocytes. Arch. Biochem. Biophys. 1999;361:75–84. doi:10.1006/abbi.1998.0959. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

