Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET
- PMID: 23578997
- PMCID: PMC4967936
- DOI: 10.2967/jnumed.112.110239
Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET
Abstract
We evaluated the ability of the PET imaging agent (89)Zr-trastuzumab to delineate HER2-positive gastric cancer and to monitor the pharmacodynamic effects of the epidermal growth factor receptor (EGFR)/human epidermal growth factor receptor 2 (HER2) tyrosine kinase inhibitor afatinib.
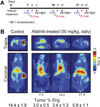
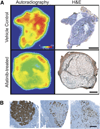
Methods: Using (89)Zr-trastuzumab, (18)F-FDG, or 3'-deoxy-3'-(18)F-fluorothymidine ((18)F-FLT PET), we imaged HER2-positive NCI-N87 and HER2-negative MKN74 gastric cancer xenografts in mice. Next, we examined the pharmacodynamic effects of afatinib in NCI-N87 xenografts using (89)Zr-trastuzumab and (18)F-FDG PET and comparing imaging results to changes in tumor size and in protein expression as monitored by Western blot and histologic studies.
Results: Although (18)F-FDG uptake in NCI-N87 tumors did not change, a decrease in (89)Zr-trastuzumab uptake was observed in the afatinib-treated versus control groups (3.0 ± 0.0 percentage injected dose per gram (%ID/g) vs. 21.0 ± 3.4 %ID/g, respectively; P < 0.05). (89)Zr-trastuzumab PET results corresponded with tumor reduction, apoptosis, and downregulation of HER2 observed on treatment with afatinib. Downregulation of total HER2, phosphorylated (p)-HER2, and p-EGFR occurred within 24 h of the first dose of afatinib, with a sustained effect over 21 d of treatment.
Conclusion: Afatinib demonstrated antitumor activity in HER2-positive gastric cancer in vivo. (89)Zr-trastuzumab PET specifically delineated HER2-positive gastric cancer and can be used to measure the pharmacodynamic effects of afatinib.
Keywords: 18F-FDG; 89Zr-trastuzumab; HER2; afatinib; gastric cancer.
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
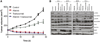

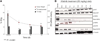
References
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. - PubMed
-
- Tafe LJ, Janjigian YY, Zaidinski M, et al. Human epidermal growth factor receptor 2 testing in gastroesophageal cancer: correlation between immunohistochemistry and fluorescence in situ hybridization. Arch Pathol Lab Med. 2011;135:1460–1465. - PubMed
-
- Hofmann M, Stoss O, Shi D, et al. Assessment of a Her2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. - PubMed
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
