Patient-reported outcomes for axitinib vs sorafenib in metastatic renal cell carcinoma: phase III (AXIS) trial
- PMID: 23579211
- PMCID: PMC3668468
- DOI: 10.1038/bjc.2013.145
Patient-reported outcomes for axitinib vs sorafenib in metastatic renal cell carcinoma: phase III (AXIS) trial
Abstract
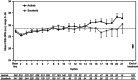
Background: Axitinib demonstrated greater progression-free survival vs sorafenib in a phase III study of previously treated patients with metastatic renal cell carcinoma. Here, we report patient-reported kidney-specific symptoms and health status, measured by the Functional Assessment of Cancer Therapy (FACT) Kidney Cancer Symptom Index (FKSI) and the European Quality of Life self-report questionnaire (EQ-5D).
Methods: In all, 723 patients received axitinib (starting dose 5 mg twice daily (b.i.d.)) or sorafenib (400 mg b.i.d.). The FKSI-15, including the disease-related symptoms (FKSI-DRS) subscale, was administered on day 1 before dosing, every 4 weeks and at end of treatment (EOT)/withdrawal. Statistical methods included a mixed-effects repeated-measures model.
Results: At baseline, patients in both arms had relatively high mean FSKI-15 and FKSI-DRS scores, comparable to the general US population. Subsequent on-treatment overall mean scores were similar between axitinib and sorafenib, and there was no substantial decline during treatment. Scores substantially worsened at EOT, mainly due to disease progression.
Conclusion: Patient-reported outcomes were comparable for second-line axitinib and sorafenib and were maintained at relatively high levels while on treatment, but worsened at EOT. As duration of treatment was longer with axitinib than sorafenib, time to worsening of symptoms can be delayed longer with axitinib.
Trial registration: ClinicalTrials.gov NCT00678392.
Figures


Comment in
-
Urological cancer: second-line option for metastatic RCC.Nat Rev Clin Oncol. 2013 Jun;10(6):305. doi: 10.1038/nrclinonc.2013.74. Epub 2013 Apr 30. Nat Rev Clin Oncol. 2013. PMID: 23629471 No abstract available.
-
Kidney cancer: AXIS trial data confirm axitinib as second-line option for mRCC.Nat Rev Urol. 2013 Jun;10(6):308. doi: 10.1038/nrurol.2013.100. Epub 2013 May 7. Nat Rev Urol. 2013. PMID: 23649292 No abstract available.
References
-
- Amir E, Seruga B, Kwong R, Tannock IF, Ocana A. Poor correlation between progression-free and overall survival in modern clinical trials: are composite endpoints the answer. Eur J Cancer. 2012;48:385–388. - PubMed
-
- Beaumont JL, Butt Z, Baladi J, Motzer RJ, Haas T, Hollaender N, Kay A, Cella D. Patient-reported outcomes in a phase III Study of everolimus versus placebo in patients with metastatic carcinoma of the kidney that has progressed on vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy. Oncologist. 2011;16:632–640. - PMC - PubMed
-
- Bukowski R, Cella D, Gondek K, Escudier B. Effects of sorafenib on symptoms and quality of life: results from a large randomized placebo-controlled study in renal cancer. Am J Clin Oncol. 2007;30:220–227. - PubMed
-
- Cella D. Quality of life in patients with metastatic renal cell carcinoma: the importance of patient-reported outcomes. Cancer Treat Rev. 2009;35:733–737. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

