Molecular mechanisms of natural killer cell activation in response to cellular stress
- PMID: 23579243
- PMCID: PMC3857624
- DOI: 10.1038/cdd.2013.26
Molecular mechanisms of natural killer cell activation in response to cellular stress
Abstract
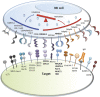
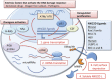
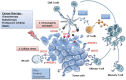
Protection against cellular stress from various sources, such as nutritional, physical, pathogenic, or oncogenic, results in the induction of both intrinsic and extrinsic cellular protection mechanisms that collectively limit the damage these insults inflict on the host. The major extrinsic protection mechanism against cellular stress is the immune system. Indeed, it has been well described that cells that are stressed due to association with viral infection or early malignant transformation can be directly sensed by the immune system, particularly natural killer (NK) cells. Although the ability of NK cells to directly recognize and respond to stressed cells is well appreciated, the mechanisms and the breadth of cell-intrinsic responses that are intimately linked with their activation are only beginning to be uncovered. This review will provide a brief introduction to NK cells and the relevant receptors and ligands involved in direct responses to cellular stress. This will be followed by an in-depth discussion surrounding the various intrinsic responses to stress that can naturally engage NK cells, and how therapeutic agents may induce specific activation of NK cells and other innate immune cells by activating cellular responses to stress.
Figures

References
-
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. - PubMed
-
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. - PubMed
-
- Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

