Menin mediates epigenetic regulation via histone H3 lysine 9 methylation
- PMID: 23579270
- PMCID: PMC3668625
- DOI: 10.1038/cddis.2013.98
Menin mediates epigenetic regulation via histone H3 lysine 9 methylation
Abstract
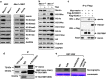
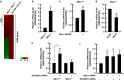
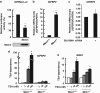
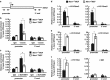
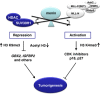
Menin, encoded by the multiple endocrine neoplasia type 1 (MEN1) gene, is a tumor suppressor that leads to multiple endocrine tumors upon loss of its function. Menin functions as a transcriptional activator by tethering MLL complex to mediate histone H3 K4 methylation. It also functions as a repressor. However, the molecular mechanism of how menin contributes to the opposite outcome in gene expression is largely unknown. Here, we investigated the role of menin in the epigenetic regulation of transcription mediated by histone covalent modification. We show that the global methylation level of histone H3 K9, as well as H3 K4, was decreased in Men1(-/-) MEF cells. Consistently, menin was able to interact with the suppressor of variegation 3-9 homolog family protein, SUV39H1, to mediate H3 K9 methylation. This interaction decreased when patient-derived MEN1 mutation was introduced into the SUV39H1-interaction domain. We show that menin mediated different chromatin changes depending on target genes. Chromatin immunoprecipitation studies showed that menin directly associated with the GBX2 promoter and menin-dependent recruitment of SUV39H1 was essential for chromatin remodeling and transcriptional regulation. These results provide a molecular basis of how menin functions as a transcriptional repressor and suggest that menin-dependent integration of H3 K9 methylation might play an important role in preventing tumors.
Figures

References
-
- Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, EmmertBuck MR, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–407. - PubMed
-
- Chandrasekharappa SC, Teh BT. Functional studies of the MEN1 gene. J Intern Med. 2003;253:606–615. - PubMed
-
- Bertolino P, Radovanovic I, Casse H, Aguzzi A, Wang ZQ, Zhang CX. Genetic ablation of the tumor suppresor menin causes lethality at mid-gestation with defects in multiple organs. Mech Dev. 2003;120:549–560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

