Oleic, linoleic and linolenic acids increase ros production by fibroblasts via NADPH oxidase activation
- PMID: 23579616
- PMCID: PMC3620266
- DOI: 10.1371/journal.pone.0058626
Oleic, linoleic and linolenic acids increase ros production by fibroblasts via NADPH oxidase activation
Abstract
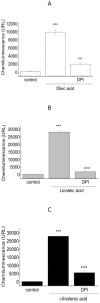
The effect of oleic, linoleic and γ-linolenic acids on ROS production by 3T3 Swiss and Rat 1 fibroblasts was investigated. Using lucigenin-amplified chemiluminescence, a dose-dependent increase in extracellular superoxide levels was observed during the treatment of fibroblasts with oleic, linoleic and γ-linolenic acids. ROS production was dependent on the addition of β-NADH or NADPH to the medium. Diphenyleneiodonium inhibited the effect of oleic, linoleic and γ-linolenic acids on fibroblast superoxide release by 79%, 92% and 82%, respectively. Increased levels of p47 (phox) phosphorylation due to fatty acid treatment were detected by Western blotting analyses of fibroblast proteins. Increased p47 (phox) mRNA expression was observed using real-time PCR. The rank order for the fatty acid stimulation of the fibroblast oxidative burst was as follows: γ-linolenic > linoleic > oleic. In conclusion, oleic, linoleic and γ-linolenic acids stimulated ROS production via activation of the NADPH oxidase enzyme complex in fibroblasts.
Conflict of interest statement
Figures




Similar articles
-
Systematic study on ROS production induced by oleic, linoleic, and gamma-linolenic acids in human and rat neutrophils.Free Radic Biol Med. 2006 Oct 1;41(7):1124-32. doi: 10.1016/j.freeradbiomed.2006.06.014. Epub 2006 Jul 4. Free Radic Biol Med. 2006. PMID: 16962937
-
Differential NADPH- versus NADH-dependent superoxide production by phagocyte-type endothelial cell NADPH oxidase.Cardiovasc Res. 2001 Dec;52(3):477-86. doi: 10.1016/s0008-6363(01)00407-2. Cardiovasc Res. 2001. PMID: 11738065
-
Volume-sensitive NADPH oxidase activity and taurine efflux in NIH3T3 mouse fibroblasts.Am J Physiol Cell Physiol. 2008 Jun;294(6):C1552-65. doi: 10.1152/ajpcell.00571.2007. Epub 2008 Apr 16. Am J Physiol Cell Physiol. 2008. PMID: 18417717
-
Responses to oleic, linoleic and α-linolenic acids in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats.J Nutr Biochem. 2013 Jul;24(7):1381-92. doi: 10.1016/j.jnutbio.2012.11.006. Epub 2013 Jan 18. J Nutr Biochem. 2013. PMID: 23333092
-
Cell-Free NADPH Oxidase Activation Assays: A Triumph of Reductionism.Methods Mol Biol. 2020;2087:325-411. doi: 10.1007/978-1-0716-0154-9_23. Methods Mol Biol. 2020. PMID: 31729001 Review.
Cited by
-
Relationship Between Composition of Fatty Acid in Platelet Phospholipid Membrane and Markers of Oxidative Stress in Healthy Men and Men After a Myocardial Infarction.Med Sci Monit Basic Res. 2021 Feb 15;27:e929634. doi: 10.12659/MSMBR.929634. Med Sci Monit Basic Res. 2021. PMID: 33583940 Free PMC article.
-
The Establishment of a Mouse Model of Recurrent Primary Dysmenorrhea.Int J Mol Sci. 2022 May 30;23(11):6128. doi: 10.3390/ijms23116128. Int J Mol Sci. 2022. PMID: 35682815 Free PMC article.
-
Circles within circles: crosstalk between protein Ser/Thr/Tyr-phosphorylation and Met oxidation.BMC Bioinformatics. 2013;14 Suppl 14(Suppl 14):S14. doi: 10.1186/1471-2105-14-S14-S14. Epub 2013 Oct 9. BMC Bioinformatics. 2013. PMID: 24267725 Free PMC article.
-
The Effect of N-Acetylcysteine on Respiratory Enzymes, ADP/ATP Ratio, Glutathione Metabolism, and Nitrosative Stress in the Salivary Gland Mitochondria of Insulin Resistant Rats.Nutrients. 2020 Feb 12;12(2):458. doi: 10.3390/nu12020458. Nutrients. 2020. PMID: 32059375 Free PMC article.
-
Gas Chromatography/Mass Spectrometry-Based Metabolomic Profiling Reveals Alterations in Mouse Plasma and Liver in Response to Fava Beans.PLoS One. 2016 Mar 16;11(3):e0151103. doi: 10.1371/journal.pone.0151103. eCollection 2016. PLoS One. 2016. PMID: 26981882 Free PMC article.
References
-
- Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313. - PubMed
-
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

