Life in the Fas lane: differential outcomes of Fas signaling
- PMID: 23579628
- PMCID: PMC11113183
- DOI: 10.1007/s00018-013-1327-z
Life in the Fas lane: differential outcomes of Fas signaling
Abstract
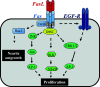
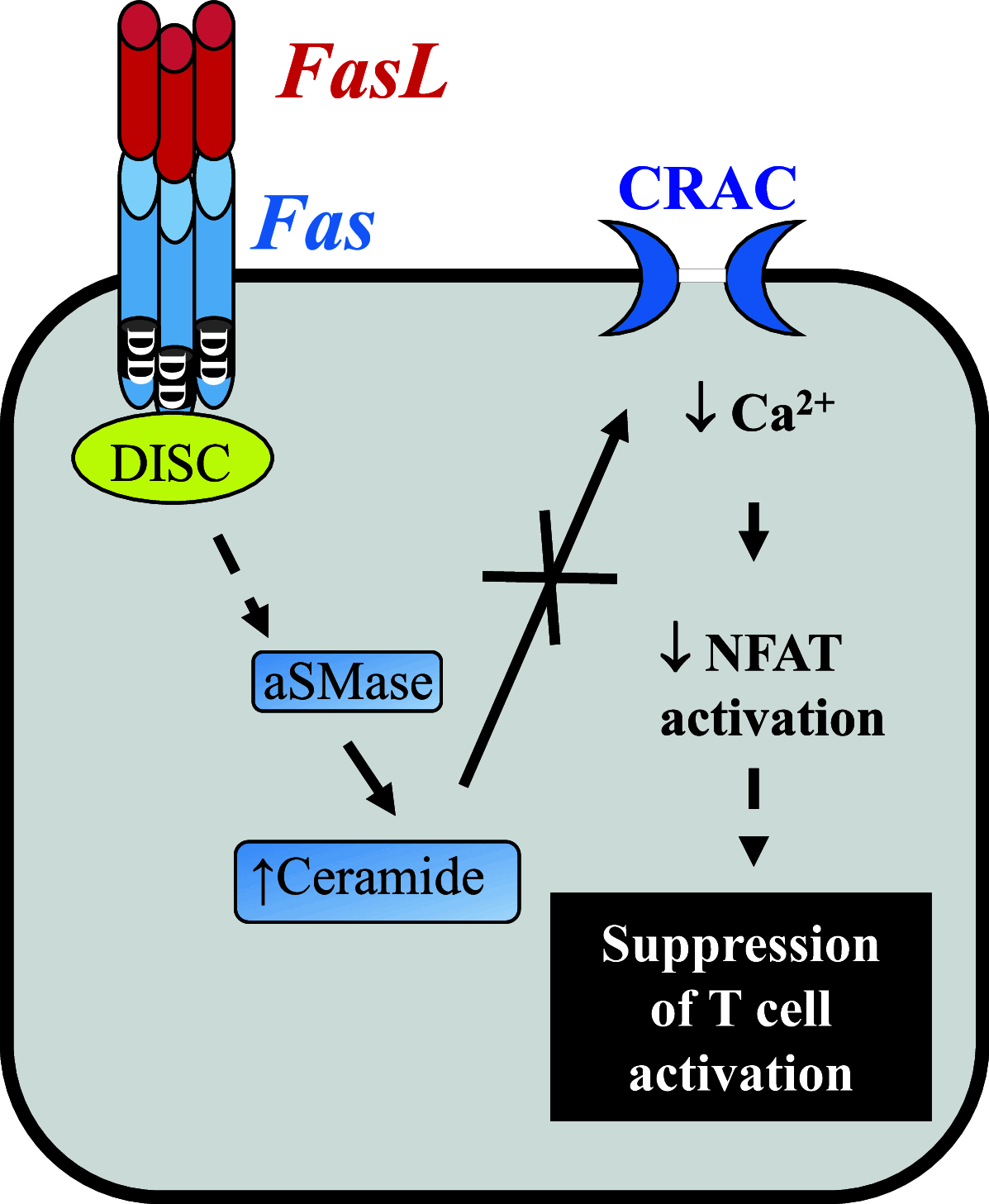
Fas, also known as CD95 or APO-1, is a member of the tumor necrosis factor/nerve growth factor superfamily. Although best characterized in terms of its apoptotic function, recent studies have identified several other cellular responses emanating from Fas. These responses include migration, invasion, inflammation, and proliferation. In this review, we focus on the diverse cellular outcomes of Fas signaling and the molecular switches identified to date that regulate its pro- and anti-apoptotic functions. Such switches occur at different levels of signal transduction, ranging from the receptor through to cross-talk with other signaling pathways. Factors identified to date including other extracellular signals, proteins recruited to the death-inducing signaling complex, and the availability of different intracellular components of signal transduction pathways. The success of therapeutically targeting Fas will require a better understanding of these pathways, as well as the regulatory mechanisms that determine cellular outcome following receptor activation.
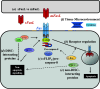
Figures

References
-
- Trauth BC, Klas C, Peters AM, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. - PubMed
-
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. - PubMed
-
- Siegel RM, Frederiksen JK, Zacharias DA, Chan FK, Johnson M, Lynch D, Tsien RY, Lenardo MJ. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. - PubMed
-
- Leithauser F, Dhein J, Mechtersheimer G, Koretz K, Bruderlein S, Henne C, Schmidt A, Debatin KM, Krammer PH, Moller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

